Abstract
PET and SPECT have suggested that there is an age-related decline of up to 10% per decade in the availability of brain serotonin transporter (SERT) in healthy subjects, starting as early as the age of 20 y. The aim of the present study was to verify these findings in young subjects. Methods: The equilibrium specific-to-nonspecific partition coefficient V″3 of the SERT ligand 11C-(+)McN5652 was obtained for 29 healthy subjects aged 18–33 y. V″3 was tested for age dependence by linear regression analysis using both a volumes-of-interest approach and voxel-based statistical parametric mapping. The sex of the subject and the season of year were considered nuisance variables. Results: Age had no significant effect on V″3. The power for the detection of an age-related decline in V″3 of the magnitude reported previously was 0.917. Conclusion: These findings indicate that age is not a relevant confounding factor for SERT availability as measured by 11C-(+)McN5652 PET in healthy adults up to the age of about 35 y.
Interest is growing in SPECT and PET of the central serotonin transporter (SERT). Not only is SERT involved in a variety of neuropsychiatric diseases, but there is also strong evidence that the density of SERT is affected by the use or abuse of various drugs—alcohol or ecstasy, for example.
In addition to sex (1) and season (2), age might be an important covariate in SPECT and PET of SERT. A pronounced age-related decline in the availability of SERT as measured by SPECT and PET has indeed been reported (Table 1).
Previous SPECT and PET In Vivo Studies on the Age Dependence of SERT Availability in Humans
For example, Hesse et al. (8), using the SPECT ligand 123I-β-2β-carbomethoxy-3β-(4-iodophenyl)tropane (CIT), reported a highly significant age-related decline of about 7%–8% per decade in the availability of SERT in SERT-rich brain regions of 22 healthy subjects. Yamamoto et al. (3), using PET with the selective SERT ligand 11C-(+)McN5652, found an age-related decline of even about 10% per decade in SERT availability in the thalamus and midbrain of 28 healthy volunteers. However, both these studies included subjects in the large age range of about 20–90 y. To our knowledge, no study has investigated age effects on SERT availability, particularly in healthy adolescents and young adults. Putative age effects on SERT availability, particularly in young subjects, are of interest in studies on drug-induced alterations of the serotonergic system, for example, because these types of studies typically include young subjects only. Inappropriate correction for an age-related decline might mask or even simulate drug effects.
Therefore, the present study investigated putative age effects on SERT availability in young, healthy subjects.
MATERIALS AND METHODS
Subjects
Twenty-nine subjects (15 female and 14 male) from the drug-naive control group of a previous study were analyzed (12). These subjects ranged from 18 to 33 y old (mean ± SD, 23.3 ± 3.7 y). Subjects were excluded from the study if they had substance-related disorders, a major acute illness, epilepsy, or an age of less than 18 y; if they were pregnant or breast feeding; or if they had a history of illicit drug abuse, major depression or schizophrenia, or alcohol, opiate, or benzodiazepine dependence. Subjects with any psychotic or mood disorder (with the exception of dysthymia) according to DSM-IV criteria (13) were also excluded. Alcohol or nicotine use that did not fulfill the DSM dependence criteria was permitted. Use of alcohol (50 ± 54 g/wk) and nicotine (13 ± 34 cigarettes per week) was moderate in the included subjects.
Participants abstained from use of any psychoactive drugs except for nicotine and caffeine for at least 3 d before the PET measurement. Abstention in this period was verified by urine or blood screening on the day of the PET examination.
PET
Dynamic PET with the SERT ligand 11C-(+)McN5652 was performed on a full-ring ECAT EXACT 921/47 system (Siemens/CTI) in 2-dimensional mode. Reconstructed images were realigned and stereotactically normalized to an 11C-(+)McN5652 template (12) using SPM99 software (Wellcome Department of Cognitive Neurology). To quantify the availability of SERT for 11C-(+)McN5652, we obtained the equilibrium specific-to-nonspecific partition coefficient V″3 (14) by applying the multilinear reference tissue method (15) (V″3 = distribution volume ratio – 1). The cerebellum was used as a reference region (16). Further details about injected tracer dose, scanning schedule, image reconstruction, stereotactic normalization, and application of the multilinear reference tissue method have been given previously (12).
Volumes of Interest
The SERT-rich brain regions mesencephalon and thalamus, in which the availability of SERT can be measured reliably with the 11C-(+)McN5652 probe, were selected for evaluation using standardized VOIs predefined in the template (12).
Statistical Analysis
The dependence of V″3 on sex (female = −1; male = 1), season (winter [October through March] = −1; summer [April through September] = 1), and age was evaluated by multiple linear regression.
In addition, univariate ANOVA of V″3 was performed using sex, season, and age as fixed factors. To simplify the interpretation of putative interaction effects, age was also reduced to 2 levels (young [age < 23 y] = −1; old [age ≥ 23 y] = 1), ending up with a 23 factorial design. The threshold of 23 y was chosen so that the 2 groups of subjects would be of similar size (young, n = 14; old, n = 15).
All tests of significance were 2 tailed. No Bonferroni adjustment for the number of VOIs was performed. SPSS software (version 10.0.7; SPSS Inc.) for Windows (Microsoft) was used.
Voxel-Based Analysis
The putative age-related decline of SERT availability was also analyzed on a voxel-by-voxel basis using the multiple-regression model of SPM99. V″3 images were smoothed with an isotropic 3-dimensional gaussian kernel of 12 mm in full width at half maximum before the voxel-based analysis. No proportional scaling was applied. The sex of the patient and the season of year were considered nuisance variables. An effect was considered statistically significant if the test reached the 1-sided significance level (α = 0.05) corrected for multiple comparisons by the standard gaussian random-field approach of SPM99.
RESULTS
Scatterplots of the partition coefficient V″3 in the mesencephalon and thalamus versus age are given in Figure 1.
Scatterplots of equilibrium specific-to-nonspecific partition coefficient V″3 in mesencephalon (A) and thalamus (B) versus subject's age.
The linear regression model was significant both in the mesencephalon (ANOVA: F2 = 3.557, P = 0.029) and in the thalamus (ANOVA: F2 = 2.994, P = 0.050). Whereas sex contributed significantly to the variance of V″3 both in the mesencephalon (β = −0.362, t = −2.148, P = 0.042) and in the thalamus (β = −0.483, t = −2.800, P = 0.010), season contributed significantly in the mesencephalon only (β = −0.424, t = −2.418, P = 0.023) (thalamus: β = −0.219, t = −1.218, P = 0.235). Age had no significant effect in either the mesencephalon (β = 0.257, t = 1.471, P = 0.154; note that the sign of β is positive, not negative as would be expected in the case of an age-related decline) or the thalamus (β = −0.044, t = −0.248, P = 0.806).
These results were validated by the univariate ANOVA. Although the model failed to reach the level of statistical significance (Table 2), significant main effects were found for sex (mesencephalon and thalamus) and season (mesencephalon). No main effect was found for age. In addition, no significant effect was found for interactions, either 2-factor or 3-factor.
Univariate ANOVA of V″3 with Sex, Season, and Age* as Fixed Factors
Voxel-based analysis of the putative correlation between V″3 and age (with sex and season as nuisance variables) did not reveal any significant effect.
DISCUSSION
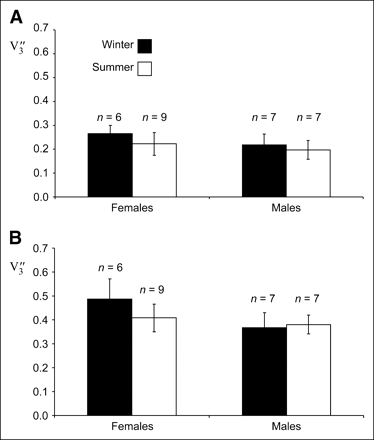
The present results indicate that SERT availability differs by sex in young, healthy subjects. SERT availability was lower in male than female subjects (Fig. 2), in agreement with previous results (1). A seasonal variation in SERT availability was found in the mesencephalon: lower in summer than in winter (Fig. 2). To some extent, this finding contradicts the results of Neumeister et al., who, using 123I-β-CIT SPECT, found an increase in thalamic or hypothalamic SERT availability in healthy female subjects in summer (no significant effect was found in the midbrain or pons in that study) (2).
Bar diagram of equilibrium specific-to-nonspecific partition coefficient V″3 in mesencephalon (A) and thalamus (B) in female and male subjects and in winter and summer (mean ± SD of sample).
No evidence was found of an age-related decline in SERT availability in the investigated young subjects. Post hoc power analysis revealed that a correlation coefficient of −0.5 between SERT availability (V″3) and age would have been detected at the α = 0.05 significance level with a power of 0.917 (G*Power, version 2.1.2 [University of Dusseldorf]: correlations, 1-tailed, sample size = 29). Therefore, at least at first sight, the present result appears to conflict not only with the results of Yamamoto et al. (3), who also performed 11C-(+)McN5652 PET, but also with all but 1 (9) previous 123I-nor-β-CIT or 123I-β-CIT studies (5–8) on healthy subjects. These studies detected significant to highly significant correlations of −0.40 to −0.80 (Table 1). However, the age range of the subjects in these studies was large—about 20–90 y—whereas the present study included only younger subjects, ranging from 18 to 33 y. This suggests that the previously reported age-related decline of SERT availability does not start until the age of about 35 y, extending the results of Dahlström et al., who, using 123I-β-CIT SPECT, did not find a correlation between age and hypothalamic or midbrain SERT availability in 41 depressive children and adolescents ranging from 7 to 17 y old (Table 1) (11).
Postmortem studies so far have failed to provide clear evidence of age-related changes in the density of SERT in the human brain, even in very old subjects (17). Therefore, one must also consider other factors that might cause the apparent age-related decline in SERT availability observed in most SPECT or PET studies so far (Table 1).
Because of the limited selectivity of the tracer used, there might be contributions of other monoamine transporters. This is particularly relevant for studies with the tracer 123I-β-CIT, which is known to bind also to the dopamine transporter (6). Apparent 123I-β-CIT uptake in SERT-rich brain regions might be affected by scattered photons from the nearby basal ganglia, which show high 123I-β-CIT uptake mainly because of high dopamine transporter density. However, the tracer 11C-(+)McN5652 is, in contrast, rather selective for SERT.
Recovery effects due to age-associated morphologic changes might also contribute to the age-related decline in SERT availability observed with SPECT or PET (5). The fact that the present study found no significant age-associated recovery effects (because there were no age effects at all) might be explained by much less pronounced morphologic changes in subjects younger than 35 y old than in older subjects (18).
Two more factors in addition to age, sex, and seasonal variations have been investigated for putative effects on SERT availability in healthy drug-naive subjects. First, in vivo regulation of SERT availability by a polymorphism in the SERT promoter gene region (5-HTTLPR), possibly modulated by a family history of axis-I disorders, has been investigated by several groups using either 123I-β-CIT SPECT (19) or 11C-(+)McN5652 PET (20). Second, variations in sex hormones, induced by cyclic changes in plasma estradiol and progesterone during the menstrual cycle, for example, might affect SERT availability (21). Neither the 5-HTTLPR genotype nor the menstrual phase at the time of PET had been determined in the subjects recruited retrospectively for the present study.
However, only 1 (19) of 5 previous studies reported that 5-HTTLPR polymorphism significantly affected SERT availability. In particular, the findings of the 11C-(+)McN5652 PET study (19) did not support the hypothesis that genotype-dependent differences in 5-HTTLPR affect SERT availability in the living human brain. Furthermore, Yamamoto et al. (3), who reported a highly significant age-related 10% decline in SERT availability as measured by 11C-(+)McN5652 PET, apparently also did not account for genotype differences. Taking all this together, it appears quite unlikely that in the present 11C-(+)McN5652 PET study, putative age effects were masked by genotype effects and therefore could not be detected.
Concerning the role of the menstrual cycle, among the 4 studies that did report a significant age-related decline in SERT availability and included women (5–8), apparently only Kuikka et al. (5) took the phase of the menstrual cycle into account (by examining women only during the first 2 wk of their cycle). The fact that 3 of these studies did find a significant age-related decline without accounting for the phase of the menstrual cycle suggests that additional variance in SERT availability caused by differences in menstrual phase was small. Therefore, it appears unlikely that in the present study, putative age effects were masked by differences in menstrual phase.
A limitation of the present study was that small, linear age effects and nonlinear changes in SERT availability over time could not be ruled out.
CONCLUSION
Our findings indicate that, as measured by 11C-(+)McN5652 PET, the age-related decline in SERT availability in SERT-rich brain regions is smaller than suggested previously in healthy adults up to the age of about 35 y, if there is any decline at all in this population. Correction for age therefore does not appear necessary in these subjects.
Acknowledgments
The study was supported by the Federal Institute for Drugs and Medical Devices (FZ: Z12.01-68503-206), Germany.
References
- Received for publication July 13, 2005.
- Accepted for publication September 19, 2005.