Abstract
Little is known about the relation between left ventricular (LV) functional reserve in response to exercise and cardiac sympathetic nervous function in patients with nonobstructive hypertrophic cardiomyopathy (HCM). We investigated whether an assessment of cardiac sympathetic nervous function by myocardial 123I-metaiodobenzylguanidine (123I-MIBG) scintigraphy might provide a sign of an abnormal LV functional reserve in response to exercise-induced β-adrenergic stimulation in patients with HCM. Methods: Thirty HCM patients underwent 123I-MIBG scintigraphy and echocardiography at rest and subsequent biventricular cardiac catheterization at rest and during dynamic exercise. LV pressures were measured using a micromanometer-tipped catheter system. The early and delayed 123I-MIBG images were quantified as a heart-to-mediastinum ratio (H/M). The plasma levels of brain natriuretic peptide (BNP) and norepinephrine (NE) were also measured. Results: Patients were divided into 2 groups according to the delayed 123I-MIBG H/M: group I consisted of 12 patients with a delayed H/M of ≤1.8 and group II had 18 patients with a delayed H/M of >1.8. Both the percentage increase from rest to exercise in LV isovolumic contraction (LV dP/dtmax) and the percentage shortening of LV pressure half-time (T1/2) as an index of isovolumic relaxation were significantly less in group I than in group II (P < 0.05, respectively). A significant linear correlation was observed between the percentage increase in LV dP/dtmax and 123I-MIBG H/Ms (early H/M: r = 0.49, P < 0.01; delayed H/M: r = 0.54, P < 0.005, respectively). A significant linear correlation was also observed between the percentage shortening in T1/2 and 123I-MIBG H/Ms (early H/M: r = 0.58, P < 0.001; delayed H/M: r = 0.64, P < 0.0005, respectively). The plasma NE levels were significantly higher in group I than in group II (P < 0.01), whereas the plasma BNP levels were comparable in the 2 HCM groups. Conclusion: β-Adrenergic enhancement of LV function during exercise may depend on the extent of cardiac sympathetic nervous innervation in HCM patients. Resting myocardial 123I-MIBG scintigraphy can noninvasively evaluate LV functional reserve in response to exercise in patients with nonobstructive HCM.
- myocardial 123I-MIBG scintigraphy
- cardiac sympathetic nervous function
- left ventricular functional reserve
- exercise
- hypertrophic cardiomyopathy
Hypertrophic cardiomyopathy (HCM) has a variety of clinical and morphologic features and is characterized by left ventricular (LV) hypertrophy and hypercontractility (1,2). This disease mainly shows impaired LV diastolic function despite well-preserved LV systolic function. Although patients with HCM have a relatively good prognosis (3–6), sudden death during exercise is a potential problem (7,8). Therefore, it is clinically relevant to determine hemodynamic changes during exercise, when β-adrenergic sympathetic tone is enhanced markedly, in patients with HCM.
The adrenergic nervous system plays a critical role in regulating physiologic functions in various cardiac diseases (9–12). Myocardial 123I-metaiodobenzylguanidine (123I-MIBG) scintigraphy is a useful imaging modality that shares similar myocardial uptake, storage, and release mechanisms as norepinephrine (NE) in the cardiac sympathetic nerve terminal. Recently, new parameters—such as measurements of a 123I-MIBG heart-to-mediastinum ratio (H/M) or washout rate—have enabled a more detailed assessment of the function and integrity of cardiac adrenergic innervation. Previous HCM studies have reported abnormal findings of 123I-MIBG, as shown by a decreased H/M or increased washout rate (13–15). In addition, 123I-MIBG scintigraphy has been considered useful in assessing the severity or prognosis of patients with HCM as well as dilated cardiomyopathy (16,17).
Recent studies have demonstrated that HCM patients show abnormal hemodynamic responses (e.g., force-frequency relation or relaxation-frequency relation) during exercise, suggesting that these hemodynamic abnormalities may be associated with an impaired LV contractile or diastolic reserve (18,19). Our previous studies have demonstrated that a progressive increase to peak heart rate (HR) in LV end-diastolic pressure (LVEDP) represents an abnormal response, whereas the biphasic changes in LVEDP during exercise show a favorable response to β-adrenergic stimulation in patients with HCM (19,20). Although the relation between cardiac sympathetic nervous dysfunction as assessed by 123I-MIBG and hemodynamic abnormalities at rest has been reported in patients with HCM (13,14), the relation between cardiac sympathetic nervous function and contractile or diastolic functional reserve during exercise remains to be investigated. Accordingly, we aimed to clarify the relation between cardiac sympathetic nervous function and LV performance in response to β-adrenergic stimulation during dynamic exercise in patients with nonobstructive HCM.
MATERIALS AND METHODS
Patient Population
Thirty patients (29 men, 1 woman; mean age, 55 ± 12 y; mean LV ejection fraction [LVEF], 69% ± 8.3%; mean ± SD) with nonobstructive HCM were enrolled in this study. HCM was diagnosed on the basis of clinical and electrocardiographic findings and echocardiographic demonstration of a hypertrophied left ventricle in the absence of other cardiac or systemic disease that itself might produce LV hypertrophy, in accordance with recently proposed diagnostic criteria (21). Patients were excluded if they had any of the following: prior evidence of myocardial infarction or coronary artery diseases with a significant stenosis of ≥50% in the major coronary artery on coronary angiography; primary valvular diseases; congestive heart failure; essential or secondary hypertension; atrial fibrillation; diabetes mellitus; neuromuscular diseases; and orthopedic problems that made exercise tests intolerable. No patients had received β-blockers, digoxin, reserpine, tricyclic antidepressant, or other drugs that could interfere with norepinephrine kinetics. Asymmetric septal hypertrophy was considered to be present if the end-diastolic thickness of the LV septum was at least 13 mm and its ratio to the thickness of the LV posterior was ≥1.3. Twenty-nine patients had asymmetric septal hypertrophy and 1 patient had concentric hypertrophy without obstruction in the LV outflow on 2-dimensional echocardiography. All patients underwent myocardial 123I-MIBG scintigraphy at rest, echocardiography at rest, and biventricular cardiac catheterization at rest and during exercise.
In addition, another age-matched control group of 5 healthy subjects (5 men; mean age, 57 ± 8 y; mean LVEF, 67% ± 5.3%; mean ± SD) also underwent echocardiography at rest and biventricular cardiac catheterization at rest and during exercise to compare the hemodynamic results with those of the HCM patients. All control subjects who had been hospitalized for suspected angina pectoris had low-risk profiles with normal cardiovascular examination results, including echocardiography, coronary angiography, and left ventriculography. No control subject was on any drug treatment for cardiac disease or had a cardiac disease that could possibly affect hemodynamic studies.
The study protocol was approved by our Institutional Review Committee. Written, informed consent was obtained from all patients.
123I-MIBG Imaging
123I-MIBG (148 MBq) was injected intravenously through an antecubital vein at rest. The planar and SPECT views were obtained approximately 15 min and 4 h after injection. A triple-head γ-camera (GCA-9300A; Toshiba Inc.) equipped with a low-energy, high-resolution collimator was rotated over a 360° arc with an acquisition time of 20 s per image at 4° intervals for each view. Energy discrimination was provided by a 20% window centered at 159 keV, and SPECT images were transferred to a computer using a 64 × 64 matrix size. No attenuation or scatter correction was applied. Tomographic slices (6-mm thick) were reconstructed using a ramp filter with a Butterworth filter (order, 8; cutoff frequency, 0.22 cycle/pixel) relative to the anatomic axis of the left ventricle. Then, vertical long-axis, horizontal long-axis, and short-axis slices were generated.
Biventricular Cardiac Catheterization and Echocardiography
Biventricular catheterization was performed in fasting patients. A 20-gauge catheter was placed in the left brachial artery to measure arterial pressure. A 6F sheath was placed in the right brachial artery and an externally balanced and calibrated 6F pigtail angiographic micromanometer-tipped catheter (model SPC-464D; Millar Instruments) was positioned in the LV cavity through the sheath to measure LV pressure. The signal from the micromanometer was adjusted to match that of the catheter. A 7F, triple-lumen Swan-Ganz thermodilution catheter (Baxter Healthcare) was positioned in the right pulmonary artery through the right subclavian vein to measure pulmonary artery wedge pressure (PAWP) and cardiac index (CI). After baseline data were obtained, patients underwent a stepwise bicycle ergometer exercise test, conducted in the supine position at an initial workload of 25 W for 3 min. The workload was increased by 25 W every 3 min until symptoms of stress appeared. Hemodynamic data were recorded during exercise.
Echocardiography was performed with a Hewlett-Packard Sonos 5500 ultrasound system equipped with a 2.5- to 3.5-MHz transducer to calculate wall thickness, cardiac chamber size, and LVEF at rest.
123I-MIBG Analysis
To evaluate regional uptake on 123I-MIBG SPECT images, the LV myocardium was divided into 5 segments (anterior, septal, inferior, lateral, and apical walls) on the midventricular level of vertical long-axis and short-axis slices. Visual evaluation regarding the presence or absence of defects in each segment was interpreted by a consensus of 3 experts who were unaware of the patient identity and clinical, echocardiographic, or catheterization data. The global cardiac uptake was quantified in anterior planar views. The region of interest (ROI) on planar imaging was manually set over the whole LV (H [heart]) and a rectangular ROI on the upper mediastinum (M) for calculating the H/M on both early and delayed images as follows: H/M = (count density of the whole LV)/(count density of the mediastinum). In our laboratory, the normal H/M values of the early and delayed 123I-MIBG images obtained from other age-matched control subjects (9 men, 1 woman; mean age, 53 ± 9 y; mean LVEF, 70% ± 7.0%; mean ± SD) were 1.9–2.8 and 1.8–2.7, respectively.
LV Functional Analysis
In the hemodynamic study, LV pressure signals were digitized at 3-ms intervals and analyzed with software developed in our laboratory using a 32-bit microcomputer system (PC-9821-ST-20; NEC Corp.). Hemodynamic data were analyzed by 2 independent observers who were unaware of the clinical, echocardiographic, and scintigraphic data. LVEDP, the maximum first derivative of LV pressure (LV dP/dtmax) as an index of contractility, and LV pressure half-time (T1/2) to evaluate LV isovolumic relaxation were measured at baseline and peak exercise as previously described (18). In right heart catheterization, PAWP and CI were also measured.
In 2-dimensional echocardiography, data were analyzed by 2 independent observers who were unaware of the clinical, hemodynamic, and scintigraphic data. LV end-diastolic dimension, LV end-systolic dimension, left atrial dimension (LAD), interventricular septal thickness (IVST), posterior wall thickness, and LVEF were measured on the M-mode of the long-axis image according to standard criteria (22).
Measurements of Neurohumoral Factors
The blood samples were collected from the left brachial artery at rest. They were immediately placed on ice and centrifuged at 4°C. The plasma NE levels were measured using high-performance liquid chromatography. The plasma brain natriuretic peptide (BNP) levels were measured with a specific radioimmunoassay for human BNP using a commercially available kit (Shionogi Co., Ltd.).
Statistical Analysis
Data are presented as mean ± SD. An unpaired t test was done to compare differences between control subjects and HCM patients. A 1-way fractional ANOVA was done to compare differences among the control group and the 2 HCM groups. Relations between continuous data were assessed using linear regression analysis. A cutoff value of a delayed H/M for detecting LV functional abnormality was defined using receiver-operating-characteristic analysis. Multiple linear regression analysis was done using the percentage increase in LV dP/dtmax and the percentage shortening in T1/2 as dependent parameters. Age, BNP, NE, 123I-MIBG H/M, IVST, and difference in double product (rate-pressure product) were used as independent parameters. The partial regression coefficient (β) was calculated to evaluate significant independent parameters. P values < 0.05 were considered statistically significant.
RESULTS
Patient Demographics and Group Classification
In the echocardiographic study, the IVST and LAD were significantly greater in HCM patients than in control subjects (IVST, P < 0.001; LAD, P < 0.01) (Table 1).
Comparison of Baseline Parameters Between Control Subjects and HCM Patients
In the hemodymanic study, the HR and mean arterial pressure (MAP) were similar in control subjects and HCM patients. The LVEDP and PAWP were significantly greater in HCM patients than in control subjects (LVEDP, P < 0.001; PAWP, P < 0.005). The T1/2 was significantly more prolonged in HCM patients than in control subjects (P < 0.01) (Table 1). Twelve patients showed a progressive increase to peak HR in LVEDP during exercise (abnormal response). In contrast, 18 patients showed the biphasic changes in LVEDP during exercise (favorable response).
In the HCM patients, the mean values of the early and delayed 123I-MIBG H/Ms were 2.0 ± 0.3 and 1.9 ± 0.3, respectively. Reduced tracer uptake was found in the inferior wall, which was more marked in the delayed images compared with the early images.
At the cutoff value of a delayed 123I-MIBG H/M of ≤1.8 using receiver-operating-characteristic analysis, the sensitivity, specificity, and accuracy for detecting HCM patients showing a progressive increase in LVEDP were 94%, 92%, and 93%, respectively. According to the quantitative 123I-MIBG findings, we then divided the HCM patients into 2 groups: group I, 12 patients with a delayed H/M of ≤1.8; and group II, 18 patients with a delayed H/M of >1.8.
Comparison of Parameters at Rest
In the echocardiographic study, the IVST and LAD were significantly greater in groups I and II than in the control group (group I: IVST, P < 0.001; LAD, P < 0.01; group II: IVST, P < 0.001; LAD, P < 0.05). The IVST and LAD were significantly greater in group I than in group II (IVST, P < 0.05; LAD, P < 0.05) (Table 1).
In the hemodynamic study, the HR and MAP did not differ significantly among the control group and the 2 HCM groups. The LVEDP and PAWP were significantly greater in groups I and II than in the control group (group I: LVEDP, P < 0.001; PAWP, P < 0.005; group II: LVEDP, P < 0.001; PAWP, P < 0.01). The LV dP/dtmax was significantly more reduced in group I than in the control group and group II (P < 0.01 vs. control group; P < 0.05 vs. group II). The T1/2 was significantly more prolonged in groups I and II than in the control group (group I: P < 0.01; group II: P < 0.01) (Table 1).
Patients in group I showed more severe and extensive defects in the inferior wall compared with those in group II.
Comparison of Parameters During Exercise
The maximum workloads at peak exercise were similar among the 3 groups. The maximum HR and the percentage increase in HR from rest to exercise were similar among the 3 groups. The peak MAP and the absolute increase in MAP from rest to exercise were similar among the 3 groups (Table 2). The peak LVEDP during exercise was significantly greater in groups I and II than in the control group (group I: P < 0.001; group II: P < 0.001). The absolute increase in LVEDP from rest to exercise was significantly greater in group I than in the control group (P < 0.05). In group II, 17 of 18 patients revealed the biphasic changes in LVEDP during exercise. In contrast, only 1 of the group I patients showed these changes. The peak LV dP/dtmax was significantly more reduced and the percentage increase in LV dP/dtmax from rest to exercise was significantly less in group I than in the control group and group II (peak LV dP/dtmax: P < 0.01 vs. control group, P < 0.01 vs. group II; % increase in LV dP/dtmax: P < 0.001 vs. control group, P < 0.001 vs. group II). The peak T1/2 was significantly more prolonged and the percentage shortening of T1/2 was significantly less in group I than in the control group and group II (peak T1/2: P < 0.05 vs. control group, P < 0.05 vs. group II; % shortening of T1/2: P < 0.05 vs. control group, P < 0.05 vs. group II). The peak PAWP and the absolute increase in PAWP (ΔPAWP) were significantly greater in groups I and II than in the control group (group I: peak PAWP, P < 0.001; ΔPAWP, P < 0.005; group II: peak PAWP, P < 0.001; ΔPAWP, P < 0.005) (Table 2).
Comparison of Parameters During Exercise and Changes in Parameters Among 3 Groups
Correlations Between 123I-MIBG and Hemodynamic Parameters
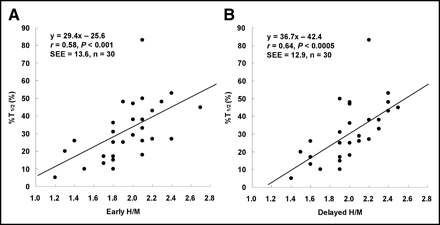
A significant positive correlation was observed between the percentage increase in LV dP/dtmax and 123I-MIBG H/M on the early as well as delayed images (r = 0.49, P < 0.01; r = 0.54, P < 0.005, respectively) (Figs. 1A and 1B). A significant positive correlation was observed between the percentage shortening in T1/2 and 123I-MIBG H/M on the early as well as delayed images (r = 0.58, P < 0.001; r = 0.64, P < 0.0005, respectively) (Figs. 2A and 2B).
Significant correlation was observed between percentage increase in LV dP/dtmax and early 123I-MIBG H/M (A) and between percentage increase in LV dP/dtmax and delayed 123I-MIBG H/M (B).
Significant correlation was observed between percentage shortening in T1/2 and early 123I-MIBG H/M (A) and between percentage shortening in T1/2 and delayed 123I-MIBG H/M (B).
Multiple regression analysis revealed that the delayed 123I-MIBG H/M was a significant independent parameter for the percentage increase in LV dP/dtmax or percentage shortening in T1/2 (β = 0.58, P = 0.02; β = 0.46, P = 0.03, respectively).
Myocardial 123I-MIBG findings and hemodynamic data of typical cases are presented in Figures 3 and 4. A patient with a normal H/M on 123I-MIBG showed a good LV functional reserve (Fig. 3), whereas another patient with abnormal 123I-MIBG findings showed an impaired LV functional reserve (Fig. 4).
123I-MIBG scintigraphy and changes in LV performance of 55-y-old man in group II. (A) Early and delayed SPECT images are almost normal except for a slightly increased washout in inferior wall (arrows). (B) Delayed planar image shows almost normal myocardial uptake. Early H/M and delayed H/M are 2.5 and 2.3, respectively. Percentage increase in LV dP/dtmax (99%) (C) and percentage shortening in T1/2 (−79%) (D) show normal responses, suggesting compatible responses of contraction and relaxation to exercise.
123I-MIBG scintigraphy and changes in LV performance of 50-y-old man in group I. (A) Increased washout of total left ventricle and defects are observed in apical and inferior walls on delayed SPECT images (arrows). (B) Delayed planar image shows markedly reduced myocardial uptake particularly in inferior wall (arrows). Early H/M and delayed H/M are 1.9 and 1.5, respectively. (C) Percentage increase in LV dP/dtmax from rest to peak exercise (44%) is decreased, suggesting a reduced contractile response to exercise. (D) Prolongation of T1/2 is found at rest. Percentage shortening in T1/2 (−24%) is decreased, suggesting impairment in shortening of relaxation.
Neurohumoral Factors
The plasma NE levels were significantly higher in group I than in group II (342 ± 77 pg/mL vs. 193 ± 71 pg/mL, P < 0.01). However, the plasma BNP levels were similar in the 2 HCM groups (70 ± 37 pg/mL vs. 59 ± 44 pg/mL, P = not significant).
DISCUSSION
In this study, LV functional reserve—as shown by the enhancement of LV contractility and relaxation by β-adrenergic stimulation during exercise—was significantly reduced in HCM patients with impaired cardiac sympathetic innervation compared with those in whom it remained intact. LV functional reserve of contractility and relaxation in response to exercise may be at least in part determined by cardiac sympathetic nervous function. Furthermore, quantitative 123I-MIBG scintigraphy rather than the plasma BNP levels may be useful in detecting the impairment in LV functional reserve of contractility and relaxation in patients with HCM.
We investigated whether the abnormal β-adrenergic regulation (e.g., impaired force-frequency relation or impaired relaxation-frequency relation) is related to cardiac sympathetic nervous dysfunction. Previous studies have suggested that β-adrenergic stimulations induced by either exercise or dobutamine infusion enhance the force-frequency and relaxation-frequency relations in normal conscious dogs (23,24). The mechanisms of adrenergic enhancement of the force-frequency and relaxation-frequency relations are thought to be related to increased Ca2+ availability to myofilaments (25). Some patients with nonobstructive HCM showed an impaired force-frequency relation during exercise despite preserved force-frequency and relaxation-frequency relations at rest, indicating that the β-adrenergic enhancement during exercise is impaired in such patients (18). We also found that patients with the biphasic changes in LVEDP during exercise can use β-adrenergic stimulation effectively, resulting in enhanced contractility and relaxation (19). Our current results further support previous results and expand their clinical implications. The force-frequency relation during exercise was less enhanced in patients with reduced cardiac sympathetic nervous function compared with those with normal sympathetic function as shown by 123I-MIBG. In this study, 17 of 18 patients with preserved cardiac sympathetic nervous function showed the biphasic changes in LVEDP during exercise, and 1 patient with reduced cardiac sympathetic nervous function showed them as well. Furthermore, the relaxation-frequency relation during exercise was less enhanced in the HCM patients with reduced sympathetic function than in those in whom it remained intact. Taken together, a vigorous β-adrenergic enhancement of LV function during exercise may depend on the extent of cardiac sympathetic nervous innervation in HCM patients.
In this study, the plasma NE levels were higher in patients with impaired sympathetic function than in those in whom it was intact, probably suggesting an inverse correlation of NE levels with sympathetic function. On the other hand, the plasma BNP levels were similar in the 2 HCM groups. The plasma BNP levels have been extensively reported to be useful in evaluating prognosis in patients with heart failure (26,27). Although the plasma BNP levels also depend on the extent of LV hypertrophy (28), our results strongly suggest that the 123I-MIBG findings, rather than the plasma BNP levels, may be useful in evaluating LV functional reserve during exercise in patients with mild-to-moderate HCM. Thus, the correlations among exercise-induced β-adrenergic enhancement of LV function, myocardial 123I-MIBG accumulation, and clinical outcomes in a larger-scale HCM population warrant further investigations.
In this study, the delayed 123I-MIBG H/M correlated better with changes in hemodynamic parameters than the early H/M. There are 2 types of 123I-MIBG uptake—neuronal uptake (uptake-1) and extraneuronal uptake (uptake-2)—and early images result from both uptake-1 and uptake-2, whereas delayed images involve less uptake-2 and represent more accurately the condition of cardiac sympathetic nervous activity itself. A previous study reported that an increase in NE turnover at cardiac sympathetic nerve endings leads to a decrease in the uptake in late images (29). Hence, the increase in turnover—that is, an increase in washout rate—is reflected in the severity of the impaired sympathetic function (29). Moreover, Nakajo et al. (30) demonstrated that the neuronal uptake of NE can be evaluated accurately if 131I-MIBG imaging is performed 4 h after 131I-MIBG administration, because the neuronal accumulation of 131I-MIBG reached a peak value 4 h after the tracer administration. Another study demonstrated that early 125I-MIBG uptake reflects only the integrity of presynaptic nerve terminals and uptake-1 function, whereas the late 125I-MIBG uptake includes overall information regarding neuronal functions from uptake to release through the storage system at nerve terminals (31). The close correlations we found between the LV functional parameters and 123I-MIBG delayed H/M rather than 123I-MIBG early H/M may further support the results of previous studies (9,29). The delayed images may reflect the severity of heart diseases more accurately than early images. Schäfers et al. (32) found an impaired uptake-1 function and β-receptor downregulation in HCM and demonstrated that β-receptor downregulation correlated with disease progression. Although we did not investigate the uptake-1 function and β-adgenergic receptor downregulation, our results may be related to their results.
It is well known that patients with HCM have a relatively good prognosis (3–6). However, sudden death during exercise is a potential problem in patients with HCM (7,8). Previous studies have documented that the risk of sudden cardiac death is significantly higher in patients with HCM who show a positive exercise test (33) or an exercise-induced abnormal blood pressure response (34). Thus, abnormal cardiac performance during exercise may contribute to sudden cardiac death. In this regard, it is relevant to assess the effects of cardiac sympathetic nervous activity on hemodynamic responses to exercise in patients with HCM. The comprehensive assessment for both β-adrenergic signaling and LV functional reserve may provide important information predicting sudden cardiac death of HCM patients.
In this study, the quantitative 123I-MIBG finding, rather than the plasma BNP levels, may be useful in detecting HCM patients with an impaired LV functional reserve. However, HCM patients with relatively low plasma BNP levels were enrolled. Thus, a closer correlation between plasma BNP levels and LV functional reserve might have been observed if we had investigated this issue in HCM patients with higher plasma BNP levels.
Differences in LV dP/dtmax response may be related to individual patient differences in plasma NE release during exercise. However, no significant differences in changes of the plasma NE levels in response to exercise were observed between patients with a good LV dP/dtmax response and those without it (18). Thus, we did not examine the changes in the plasma NE levels during exercise in this study.
We classified the HCM patients only using a cutoff value of the delayed 123I-MIBG H/M. Although the early H/M and washout rate are also useful in evaluating heart disease states, determination of which parameters are the most useful in evaluating them is still controversial. We concur with the results of Sisson et al. (31), who showed that the delayed 125I-MIBG uptake includes overall information regarding neuronal function from uptake to release through the storage system at nerve terminals. Therefore, we have used the delayed H/M for patient classification. Because we did not measure the washout rate in all patients, we did not apply it.
CONCLUSION
Resting myocardial 123I-MIBG scintigraphy can noninvasively evaluate LV functional reserve in response to exercise in patients with nonobstructive HCM. The assessment of cardiac sympathetic nervous function, rather than the plasma BNP levels, may be more useful in detecting patients with an impaired LV functional reserve in HCM.
Acknowledgments
We thank Shinji Abe, Satoshi Nakano, and Masanari Nishino, radiologist, for their technical assistance with the 123I-MIBG scingraphic study.
Footnotes
Received Sep. 30, 2004; revision accepted Feb. 16, 2005.
For correspondence or reprints contact: Satoshi Isobe, MD, PhD, Department of Cardiology, Nagoya University Graduate School of Medicine, 65 Tsurumai-cho, Showa-ku, Nagoya, Aichi 466-8550, Japan.
E-mail: sisobe{at}med.nagoya-u.ac.jp