Abstract
Left ventricular (LV) remodeling after myocardial infarction (MI) is a maladaptive process that increases the risk of heart failure and death. The myocardial phosphoinositide cycle, which is located downstream from several neurohumoral factors, plays a crucial role in LV remodeling. Our animal studies demonstrated that 1-[1-11C]butyryl-2-palmitoyl-rac-glycerol (11C-DAG) can be used to visualize regions with an activated phosphoinositide cycle. Therefore, we examined whether myocardial 11C-DAG accumulation assessed by PET is relevant to LV enlargement and systolic dysfunction in post-MI patients. Methods: We performed PET with 11C-DAG in 13 post-anteroseptal MI patients and 4 healthy volunteers. We placed regions of interest on the noninfarcted myocardium and calculated the myocardium-to-left atrial (LA) chamber ratio of 11C-DAG accumulation. Results: The myocardium-to-LA chamber ratio of 11C-DAG was significantly higher in the post-MI patients (mean ± SD, 1.73 ± 0.35) compared with that of the healthy volunteers (mean ± SD, 1.25 ± 0.13; P < 0.05). In the post-MI patients, the myocardium-to-LA chamber ratio of 11C-DAG was significantly correlated with the LV end-diastolic volume index (r = 0.79, P < 0.01) and the plasma concentration of brain natriuretic peptide (r = 0.85, P < 0.001) and negatively correlated with the LV ejection fraction (r = −0.69, P < 0.01). Conclusion: These findings suggest that the myocardial 11C-DAG accumulation assessed by PET is relevant to LV enlargement, LV systolic dysfunction, and humoral activation in post-MI patients. This new imaging strategy based on intracellular signaling may contribute to the assessment and treatment of post-MI patients.
Left ventricular (LV) remodeling, the expansion of the infarct zone and characteristic changes in the noninfarcted myocardium (e.g., cardiomyocyte hypertrophy, interstitial fibrosis, and impaired contraction and relaxation, etc.), is a maladaptive process that causes deterioration of the LV function and increases the risk of congestive heart failure as well as cardiovascular death after myocardial infarction (MI) (1). The Survival and Ventricular Enlargement (SAVE) trial and other randomized clinical trials have shown that angiotensin-converting enzyme inhibitors prevent LV remodeling and the worsening of heart failure and decrease cardiovascular death in post-MI patients (2–5). The results of these trials strongly suggest that the rennin–angiotensin system plays a pivotal role in the development of LV remodeling and the resultant worsening of heart failure in post-MI patients.
The phosphoinositide cycle is an intracellular signal transduction system located at the downstream pathways of Gqα protein and phospholipase Cβ1, which couples with angiotensin II, endothelin-1, and α1-adrenergic receptors (6), and plays a key role in LV remodeling after MI. One of the important second messengers of the phosphoinositide cycle is diacylglycerol. Diacylglycerol activates protein kinase C, which has been shown to play an important isozyme-specific role in the development of cardiac hypertrophy and failure (7–10). Therefore, noninvasive assessment of the myocardial phosphoinositide cycle in post-MI patients might provide a useful tool to assess the development of LV remodeling and might contribute to optimizing treatments that ameliorate post-MI LV remodeling and heart failure.
We previously reported that intravenously administered 1-[1-11C]butyryl-2-palmitoyl-rac-glycerol (11C-DAG), a 11C-labeled diacylglycerol, was metabolized to the intermediates of the phosphoinositide cycle in the rat brain and that cholinergic stimulation prompted the accumulation of 11C-DAG (11). We also reported that intravenously administered 11C-DAG was also predominantly metabolized to the intermediates of the phosphoinositide cycle in the rat infarcted heart and enabled successful visualization of the myocardial region with the activated phosphoinositide cycle (12). Furthermore, we demonstrated that the angiotensin-converting enzyme inhibitor captopril suppressed the accelerated myocardial 11C-DAG accumulation in the infarcted rat heart (13). The results of these previous studies show that accelerated myocardial accumulation of 11C-DAG reflects the activated phosphoinositide cycle. It is unclear whether the myocardial phosphoinositide cycle also plays a key role in post-MI LV remodeling in humans. In the present study, we investigated the hypothesis that myocardial accumulation of 11C-DAG assessed by PET is relevant to LV enlargement and LV systolic dysfunction in post-MI patients.
MATERIALS AND METHODS
Patients
We studied 13 patients (7 men; mean age ± SD, 68 ± 7 y) with LV dysfunction due to previous anteroseptal Q-wave MI 4–53 mo after MI. The clinical characteristics of the patients are shown in Table 1. The diagnosis of MI was based on a typical medical history, electrocardiographic changes, and biochemical detection of cardiac enzyme release, although the maximum creatine kinase level was uncertain in 3 patients. Eight patients underwent primary percutaneous coronary intervention for the left anterior descending coronary artery, resulting in thrombolysis in myocardial infarction (TIMI) grade III of the coronary flow. In one patient, rescue percutaneous coronary intervention followed unsuccessful coronary thrombolysis. In 4 patients, percutaneous coronary intervention for the residual narrowing or occlusion of the left anterior descending coronary artery was performed in the chronic phase because spontaneous recanalization had occurred in the acute phase or they had not been considered as candidates for the reperfusion therapy in the acute phase. No patients had a significant coronary artery stenosis in the right or left circumflex coronary arteries. All post-MI patients were New York Heart Association functional class 1 or 2, and none had an episode of worsening of heart failure requiring hospital admission within 3 mo of the PET study. Nine post-MI patients had been treated with angiotensin-converting enzyme inhibitors and one post-MI patient had been treated with angiotensin II type 1 receptor antagonist. Seven post-MI patients had been treated with β-blockers. Plasma brain natriuretic peptide (BNP) concentrations were assessed with a highly sensitive radioimmunoassay kit (Shionoria BNP; Shionogi) 17 ± 18 mo (mean ± SD) after the onset of acute MI when they were in a very stable condition. We also studied 4 healthy volunteers (mean age ± SD, 26 ± 5 y). None of the healthy volunteers had abnormal echocardiographic findings. The purpose and nature of the present study were approved by the ethics committees of our institutes. Written informed consent was obtained from every subject before the study.
Clinical Characteristics and Myocardium/LA Chamber Ratio of 11C-DAG of Post-MI Patients and Healthy Volunteers
Echocardiography and Cardiac Catheterization
We performed echocardiography in all patients and healthy volunteers. Examinations were performed while the subjects were in the left lateral recumbent position. Standard echocardiographic views of the LV were obtained, and end-diastolic and end-systolic dimensions and wall thickness of the interventricular septum and the LV posterior wall were determined. LV dimensions were measured according to the recommendations of the American Society of Echocardiography (14).
We performed cardiac catheterization in all post-MI patients within 5.2 ± 7.0 mo (mean ± SD) after the onset of MI when the patients were in a very stable condition. The biplane LV angiograms were filmed using right and left anterior oblique views, and the LV end-diastolic and end-systolic volumes were estimated using the area–length method (15).
Synthesis of 11C-DAG
11C-DAG was synthesized as previously described in detail (11,16). Briefly, 11C-ethylketene, which was synthesized using a cyclotron, was trapped in a pyridine solution containing 1 μmol/L 2-palmitoylglycerol. The acylation reaction was performed using 11C-ethylketene under no-carrier-added conditions. 11C-DAG had a high specific activity of 186 GBq/μmol at the end of synthesis. Radiochemical purity of 11C-DAG was 98% at the time of injection. Human application and quality control of 11C-DAG followed the guidelines established by the Committee of PET in Nishijin Hospital (Kyoto, Japan).
PET
We performed PET in post-MI patients (20 ± 16 mo [mean ± SD] after the onset of acute MI) and healthy volunteers using a Headtome IV (Shimadzu; in-plane full width at half maximum [FWHM], 4.5 mm; axial FWHM, 11.0 mm; axial field of view, 9 cm; number of planes, 21). After transmission scanning to correct attenuation, we first performed PET after the inhalation of 15O-carbon monoxide to visualize the left atrial (LA) and LV chambers. After the 15O radioactivity in the blood pool returned to the baseline level, we injected 1,110 MBq of 11C-DAG intravenously. Since the 11C radioactivity in the blood pool became sufficiently low to allow appropriate contrast between the blood pool and noninfarcted myocardium approximately 20 min after the 11C-DAG injection, we collected static data from 15 to 27 min after the injection. We generated the short axial slices of the heart (matrix size, 128 × 128; pixel size, 2 mm; slice thickness, 6 mm) and placed 12–18 circular regions of interest (ROIs) (diameter, 11.0 mm) on the noninfarcted myocardium (the inferior and lateral wall of the LV) using 3 basal short axial slices in each subject. We also placed 4–6 circular ROIs (diameter, 11.0 mm) in the LA chamber using 2 short axial slices of the LA. We did not correct the partial-volume effect on the recovery of counts in the present study. Then, we calculated the myocardium-to-LA chamber ratio of 11C-DAG accumulation by dividing the average counts of 11C-DAG in the inferior and lateral wall by the counts of 11C-DAG in the LA chamber.
Statistical Analysis
All values are expressed as mean ± SD. Statistical analysis of differences between groups was done by the unpaired Student t test. A correlation between the 2 parameters was determined by simple linear regression analysis. Statistical significance was accepted at a level of P < 0.05.
RESULTS
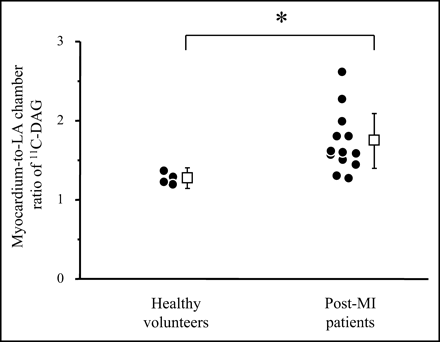
The clinical characteristics of the post-MI patients and the healthy volunteers are shown in Table 1. Figure 1 shows the short axial slices of PET with 11C-DAG of a healthy volunteer and 2 post-MI patients (patients 6 and 4). The myocardium-to-LA chamber ratio of 11C-DAG was significantly increased in the post-MI patients (1.73 ± 0.35) compared with that of the healthy volunteers (1.25 ± 0.13, P < 0.05; Fig. 2). In the post-MI patients, the myocardium-to-LA chamber ratio of 11C-DAG significantly correlated with the LV end-diastolic volume index (r = 0.79, P < 0.01; Fig. 3A) and the plasma concentration of BNP (r = 0.85, P < 0.001; Fig. 3C) and negatively correlated with the LV ejection fraction (r = −0.69, P < 0.01; Fig. 3B). Although we had one patient with a highly increased BNP level, the correlation between the myocardium-to-LA chamber ratio of 11C-DAG and the BNP level was still significant after excluding this patient (r = 0.64, P < 0.05).
Representative short axial slices of PET with 11C-DAG in healthy volunteer (A) and 2 post-MI patients (B and C). In healthy volunteer, myocardial accumulation of 11C-DAG was mild and myocardium-to-LA chamber ratio of 11C-DAG was 1.28 (A). In post-MI patient 6, myocardial accumulation of 11C-DAG was also mild and myocardium-to-LA chamber ratio of 11C-DAG was 1.39 (B). In patient 4, a remarkable accumulation of 11C-DAG was observed in remote viable myocardium (inferior septum and inferolateral wall). Myocardium-to-LA chamber ratio of 11C-DAG was 2.58 (C).
Myocardium-to-LA chamber ratio of 11C-DAG in healthy volunteers and post-MI patients. *P < 0.05.
Correlations between myocardium-to-LA chamber ratio of 11C-DAG and LVEDVI (A), LVEF (B), and plasma concentration of BNP (C). LVEDVI = left ventricular end-diastolic volume index; LVEF = left ventricular ejection fraction.
Possibly, the difference in the wall thickness among the subjects might have affected the results demonstrated in Figures 2 and 3 through the partial-volume effect. However, the wall thickness of post-MI patients determined by echocardiography was not significantly increased compared with that of the healthy volunteers in both the interventricular septum (8.6 ± 1.5 vs. 7.7 ± 0.5 mm, respectively) and the LV posterolateral wall (9.3 ± 1.3 vs. 8.0 ± 0.8 mm, respectively). Furthermore, the myocardium-to-LA chamber ratio of 11C-DAG did not correlate with the myocardial wall thickness of the LV posterior wall (r = 0.11, P = 0.73) that were determined by echocardiography.
DISCUSSION
In the present study, we demonstrated that the myocardial accumulation of 11C-DAG in the remote viable myocardium of post-MI patients was significantly increased compared with that of the healthy volunteers. Furthermore, the accumulation of 11C-DAG in the post-MI patients significantly correlated with the LV enlargement, LV systolic dysfunction, and plasma BNP levels.
Mechanism of 11C-DAG Accumulation
In our previous studies (11,17,18), we reported that intravenously administered 11C-DAG was metabolized to the intermediates of the phosphoinositide cycle—that is, phosphatidic acids, phosphatidylinositols, and phosphatidylinositol phosphates in the rat brain—and that cholinergic stimulation promoted the accumulation of 11C radioactivity. These results suggest that 11C-DAG accumulation in the rat brain reflects the phosphoinositide metabolic process, which is stimulated by cholinergic neurotransmission (11). Furthermore, in our recent study (12), we administered 11C-DAG intravenously to rats 7 d after MI to investigate the metabolic fate of 11C-DAG in myocardium. In that study, thin-layer chromatography of 11C-DAG metabolites obtained from both infracted and noninfarcted myocardium revealed that intermediates of the phosphoinositide cycle were the predominant metabolites in both infarcted and noninfarcted myocardium (12). In our more recent study using the same acute MI model in rats, the accelerated myocardial 11C-DAG accumulation 3 wk after the onset of MI was significantly suppressed by a 3-wk captopril treatment that was initiated immediately after the onset of MI (13). These results from our previous animal studies suggest that intravenously administered 11C-DAG was predominantly metabolized to intermediates of the phosphoinositide cycle and that myocardial accumulation of 11C-DAG depends on the activity of the phosphoinositide cycle, which is located downstream of angiotensin II, endothelin-1, and α1-adrenergic receptors.
Myocardial 11C-DAG Accumulation and LV Remodeling
As shown in Figure 2, the myocardial accumulation of 11C-DAG in the post-MI patients was relatively scattered. As shown in Table 1, LV end-diastolic volume was not enlarged and LV ejection fraction was well preserved in some post-MI patients, presumably due to early coronary reperfusion performed in these patients. In fact, the average myocardium-to-LA chamber ratio of 11C-DAG of 6 post-MI patients whose LV end-diastolic volume index was <80 mL/m2 was 1.45 ± 0.13. This value was not significantly different from those of the healthy volunteers. We then investigated the relationship between myocardial 11C-DAG accumulation and LV end-diastolic volume, LV ejection fraction, and plasma BNP level in post-MI patients and found that the myocardial accumulation of 11C-DAG correlated with these parameters. The results of the present study, in conjunction with those of our previous animal studies, suggest that PET with 11C-DAG in post-MI patients can be used to visualize the myocardial phosphoinositide cycle that is activated in the process of LV remodeling, providing a useful tool for noninvasive assessment of the downstream signaling pathway of neurohumoral factors such as angiotensin II, endothelin-1, and norepinephrine.
The results of our present study are in agreement with several earlier reports demonstrating that the phosphoinositide cycle as well as its upstream and downstream pathways may contribute greatly to the development of cardiac hypertrophy and failure in animal models. Ju et al. demonstrated that the myocardial Gqα protein and phospholipase Cβ1 pathways, located upstream of the phosphoinositide cycle, was upregulated in a rat model of MI (19). Kawaguchi et al. reported that myocardial accumulation of inositol 1,4,5-trisphosphate and diacylglycerol, both of which are second messengers of the phosphoinositide cycle, significantly increased in isolated cardiac myocytes from spontaneously hypertensive rats (20). It was unknown, however, whether the phosphoinositide cycle plays a crucial role in humans in the development of post-MI LV remodeling, cardiac hypertrophy, and heart failure because no strategy for in vivo assessment of the myocardial phosphoinositide cycle was available. In this regard, we believe that PET with 11C-DAG provides a new tool to assess the myocardial phosphoinositide cycle in humans in vivo.
Discrepancy Between Animal and Human Studies
In our previous studies (12) using quantitative autoradiography in rats 3 wk after MI, intravenously administered 11C-DAG accumulated in both infarcted and noninfarcted myocardium. The 11C-DAG accumulation in the infarcted myocardium, however, was not visualized in the present study. In our previous animal experiment, the accelerated 11C-DAG accumulation in the infarcted myocardium 3 wk after the onset of MI was associated with abundant inflammatory cells, such as macrophages and myofibroblasts. Furthermore, 11C-DAG uptake in the infarcted myocardium in rats significantly decreased 10 wk after MI, and this decrease was associated with the decreased number of inflammatory cells (13). Since we investigated the post-MI patients 20 ± 16 mo after the onset of MI in the present study, the healing process in the infarcted myocardium may have been almost completed, and there may have been few inflammatory cells in the scar tissue. The difference in the time points between the previous animal experiments and the present clinical study, therefore, may explain the difference in the 11C-DAG uptake in the infarcted myocardium. Furthermore, the limited spatial resolution of PET compared with that of autoradiography may have attenuated the recovery of 11C-DAG counts in the infarcted myocardium because of the decreased thickness of the scar tissue compared with the viable remote myocardium. Therefore, the difference in the spatial resolution of the modality may have contributed partially at least to the difference in 11C-DAG accumulation in the infarcted myocardium between the previous animal studies and the present clinical study.
Myocardial 11C-DAG Accumulation in Noninfarcted Heart
In the present study, mild accumulation of 11C-DAG was observed in myocardium of the healthy volunteers. An in vitro experimental study by Steinberg and Alter demonstrated that cardiac myocytes accumulate inositol phosphates even at the baseline condition (21). Although the crucial roles of the myocardial phosphoinositide cycle in pathologic conditions have been established (22–24), it is unclear how the phosphoinositide cycle participates in the maintenance of homeostasis in the cardiovascular system. Further study is needed to clarify the implications of the mild 11C-DAG accumulation in the myocardium of the healthy volunteers.
Myocardial 11C-DAG Accumulation and Medication
Although most post-MI patients in the present study had been treated with angiotensin-converting enzyme inhibitors or angiotensin receptor blockers, the myocardial accumulation of 11C-DAG in some patients was still high. There are 2 possible explanations regarding this issue. First, as shown in Table 1, the dose of medication to suppress the renin–angiotensin system might not have been sufficiently high, and no patients received both angiotensin-converting enzyme inhibitors and angiotensin receptor blockers. The medication in some post-MI patients might not have been sufficient to reduce the myocardial accumulation of 11C-DAG since an additional analysis showed that the myocardium-to-LA chamber ratio of 11C-DAG was not significantly different between the post-MI patients treated with and without angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers (1.80 ± 0.36 vs. 1.48 ± 0.08, respectively; P = 0.16). Therefore, it would be very interesting to assess the myocardial 11C-DAG accumulation after increasing the dose of angiotensin-converting enzyme inhibitors or angiotensin receptor blockers or after adding either of these drugs in patients for whom they were not initially prescribed. Second, the myocardial phosphoinositide cycle has been shown to mediate signaling not only from angiotensin II but also from endothelin-1 and α1-adrenergic receptor agonists (6). The effects of blockades for endothelin-1 and α1-adrenergic agonists should also be investigated.
Limitations
Some limitations of this study should be mentioned:
Because we did not correct for the partial-volume effect on myocardial 11C-DAG counts, it is possible that the high myocardial 11C-DAG accumulation shown in some post-MI patients was due to the increased wall thickness. This would be unlikely, however, because the myocardium-to-LA chamber ratio of 11C-DAG did not correlate with the ventricular wall thickness as described earlier. Furthermore, the wall thickness of the post-MI patients was not significantly increased compared with that of the healthy volunteers, as also demonstrated earlier. Therefore, the partial-volume effect would not likely explain the increased myocardium-to-LA chamber ratio of 11C-DAG accumulation in the post-MI patients.
The variation in the period after the onset of MI in our patients might have had an impact on the LV remodeling, as would the difference in the initial extent of myocardial infarction. However, the period after the onset of MI was not significantly correlated with the myocardium-to-LA chamber ratio of 11C-DAG in the present study (r = 0.48, P = 0.10). Therefore, the myocardial accumulation of 11C-DAG could not be simply explained by the variation in the time after MI in our patient group.
In the present study, we could not assess the metabolic fate of 11C-DAG that accumulated in the myocardium of the study patients because myocardial biopsy was not performed after the injection of 11C-DAG. In our previous animal study (12), although the intermediates of the phosphoinositide cycle were a predominant metabolic fate of 11C-DAG, we also found 11C radioactivity in butyryl CoA, phosphatidylcholine, and phosphatidylethanolamine, which are not intermediates of the phosphoinositide cycle. Further studies, including myocardial biopsy after the 11C-DAG injection, is required to determine the metabolic fate of 11C-DAG that accumulates in the human myocardium.
CONCLUSION
Although we need further studies with a larger population to establish the clinical significance of PET with 11C-DAG in post-MI patients, the results of the present study suggest that myocardial accumulation of 11C-DAG assessed by PET in post-MI patients is related to LV enlargement, LV systolic dysfunction, and the plasma BNP level. This new imaging strategy based on intracellular signaling may contribute to optimizing the choice of therapies to prevent LV remodeling and subsequent heart failure in post-MI patients.
Acknowledgments
We thank Hitoshi Horii and Shoichi Watanuki for their support with the tomographic studies and Brent Bell for reading the manuscript. This work was funded by Grants-in-Aid for Science Research (13670687 and 15390441) from the Ministry of Education, Science, Sports and Culture of Japan. This work was also funded by a Japan Heart Foundation/Pfizer Pharmaceuticals Inc. Grant for Research on Cardiovascular Disease.
Footnotes
Received Jun. 16, 2004; revision accepted Dec. 8, 2004.
For correspondence or reprints contact: Yutaka Kagaya, MD, PhD, Department of Cardiovascular Medicine, Tohoku University Graduate School of Medicine, 1-1 Seiryomachi, Aobaku, Sendai, 980-8574, Japan.
E-mail: kagaya{at}cardio.med.tohoku.ac.jp