Abstract
131I-labeled metaiodobenzylguanidine (MIBG) is an established treatment modality for neuroendocrine tumors. Because of low tumor doses, it has a predominantly palliative character. Our approach was to investigate whether intraarterial application of 131I-MIBG has the potential to enhance tumor uptake. Methods: Seventeen patients with primary or metastasized neuroendocrine tumors received intraarterial treatment with 131I-MIBG, and 12 of these patients also had intravenous treatment. Every patient underwent intravenous 131I-MIBG whole-body scanning before therapy. For quantification, a tumor–to–whole-body ratio was calculated from the diagnostic and 24-h posttreatment scans. Results: Compared with the intravenous application, intraarterial 131I-MIBG treatment provided an up to 4-fold higher tumor uptake. Mean uptake was enhanced by 69%, but this varied widely between patients. We did not observe any immediate complications from catheterization. Carcinoid-related side effects were noted in 7 of 17 patients and were not different from those seen with intravenous application. Conclusion: Intraarterial treatment with 131I-MIBG is a safe alternative to intravenous application and provides a 69% higher mean tumor uptake. We propose to attempt intraarterial MIBG treatment in every patient to assess its potential benefit.
- 3-iodobenzylguanidine
- iodine radioisotopes
- neuroendocrine tumors
- radioisotope therapy
- intraarterial infusions
Metaiodobenzylguanidine (MIBG) labeled with 131I is an established treatment modality for neuroendocrine tumors. Its therapeutic effectiveness has been proven in pheochromocytomas, neuroblastomas, paragangliomas, and carcinoid tumors (1–4). Because of the predominantly low tumor dose that can be achieved, treatment is mostly palliative and objective response is rare. Various efforts have been made to overcome this problem, including increasing the injected activity, using non–carrier-added MIBG, or pretargeting with cold MIBG (5). Selective intraarterial application of 131I-MIBG may be an alternative way to increase dose to the tumor. The aim of this study was to investigate whether intraarterial infusion of 131I-MIBG enhances tumor uptake and whether there are any relevant side effects.
MATERIALS AND METHODS
Between May 1996 and March 2002, 17 patients (12 men and 5 women; mean age, 60.3 y) underwent intraarterial treatment with 131I-MIBG. Sixteen patients had carcinoid tumors (primary or metastatic), and 1 patient had a paraganglioma. Twelve patients received both intravenous and intraarterial treatment with 131I-MIBG, and 5 patients received only the intraarterial infusion (Table 1). Patients underwent follow-up CT for a mean of 43 mo (range, 0–72 mo).
Patient Details
131I-MIBG of high specific activity (>1.11 MBq/mg, Nycomed-Amersham) was used both for diagnostic scanning and for treatment. To demonstrate therapeutically relevant tumor uptake, pretreatment 131I-MIBG whole-body scanning was performed 24 h after intravenous application of 350–400 MBq using a double-head γ-camera (Genesis; ADAC Laboratories) equipped with high-energy collimators. The energy window was set to 364 keV ± 20%, matrix size was set to 512 × 512 × 16, and scan speed was 8 cm/min. The counting-rate performance of the γ-camera was checked using a 131I source surrounded by paraffin to simulate attenuation and scatter. A linear relationship between activity and counting rate was found for activities of up to 2,500 MBq in the field of view, with a dead-time loss of <10%.
For intraarterial therapy, a femoral artery catheter was placed at angiography (Integris 2000; Philips). In cases of liver metastases, the catheter was placed in the common hepatic artery. In extrahepatic tumors, the supplying artery (the superior mesenteric artery in 4 patients, a branch of the ileocolic artery in 1 patient, and the fourth lumbar branch in 1 patient) was accessed through the abdominal aorta or celiac trunk and was imaged, and a catheter was placed there. 99mTc-labeled macroaggregated albumin (100 MBq) was injected via the catheter, and a planar image of the abdomen was acquired 5 min later to verify that the catheter was positioned correctly after transfer of the patient to the nuclear medicine department.
Treatment was administered as an intravenous or intraarterial infusion of 3,500–4,000 MBq of 131I-MIBG over 30 min. Anterior views of the diagnostic and 24-h posttreatment scans were used for quantification. Irregularly shaped regions of interest were drawn for the whole body (excluding tumor) and the tumor, and a ratio of tumor to whole body was calculated. For calculation of such a ratio, the use of anterior views provides information equivalent to that obtained geometrically. The size and shape of the regions of interest for each patient were kept constant in all scans, but their position was adjusted if necessary.
RESULTS
Tumor uptake of MIBG therapy was up to 4-fold higher (mean, 1.7-fold) for the intraarterial application than for the standard intravenous application (Table 2). For those 12 patients who received both intravenous and intraarterial therapy, the mean tumor–to–whole-body ratios were 4.11 ± 3.66 and 6.45 ± 5.44, respectively. This difference was statistically significant (paired t test, P = 0.0033). The enhancement of tumor uptake was not significantly different between liver metastases and tumors in other locations (t test, P = 0.16). Furthermore, the whole-body distribution of 131I-MIBG did not differ between intravenous and intraarterial tracer applications; in particular, no increased bowel activity was seen with intraarterial application. Figure 1 demonstrates that the improved uptake with intraarterial therapy is achieved regardless of whether patients received intraarterial or intravenous treatment first.
Comparison of tumor uptake in patients who first received intraarterial treatment with patients who first received intravenous treatment.
Ratio of Tumor Activity to Whole-Body Activity at 24 Hours After Injection
Figures 2–4 show images of 3 different patients in whom intraarterial treatment led to a higher tumor uptake in liver metastases and in an extrahepatic tumor.
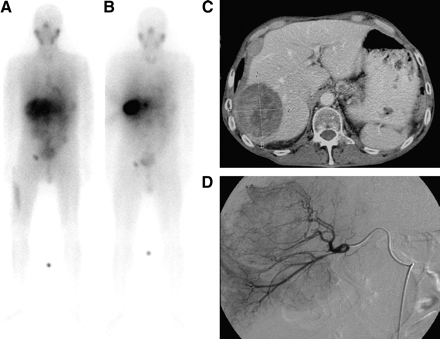
Images of 54-y-old man (patient 3 in Tables 1 and 2) who was diagnosed in 1986 with carcinoid of appendix, underwent appendectomy in July 1986, and received chemotherapy from July to November 1997. (A) 131I-MIBG whole-body scan obtained in November 1999 after intravenous treatment shows good uptake in a large liver metastasis. (B) 131I-MIBG whole-body scan obtained in April 1998 after intraarterial treatment shows better uptake and less abdominal and background activity than after intravenous application. (C) CT scan obtained in April 1998 shows a large liver metastasis. (D) Angiogram obtained in April 1998 shows the hypervascularized liver metastasis.
Images of 44-y-old man (patient 12 in Tables 1 and 2) who was diagnosed in 1998 with inoperable carcinoid of pancreas with infiltration of mesenteric root. He underwent hepaticojejunostomy and 4 MIBG treatments between February 1999 and March 2000. (A) 131I-MIBG whole-body scan obtained in February 1999 after intravenous treatment shows poor uptake in the carcinoid of pancreas. (B) 131I-MIBG whole-body scan obtained in June 1999 after intraarterial treatment shows good focal uptake in the carcinoid of pancreas. Treatment followed selective catheterization of superior mesenteric artery. (C) CT scan obtained in June 1999 shows the carcinoid of pancreas. (D) Angiogram obtained in June 1999 shows the tumor-supplying arteries.
Images of 61-y-old man (patient 9 in Tables 1 and 2) who was diagnosed with liver metastases of carcinoid in 1998. Primary tumor could not be located by imaging and laparotomy. (A) 131I-MIBG whole-body scan obtained in November 1999 before intravenous treatment shows reasonably good uptake in the liver metastasis, but there is considerable background activity. (B) 131I-MIBG whole-body scan obtained in June 1999 after intraarterial treatment again shows good uptake in the liver metastasis, with much less background activity. (C) CT scan obtained in June 1999 shows the liver metastasis. (D) Angiogram obtained in June 1999 shows the tumor-supplying arteries.
Catheterization caused no acute complications. Carcinoid-related side effects (hypertension, vomiting, and hyperhidrosis) were observed in 7 of 17 patients. In 1 of these patients, a malignant carcinoid syndrome developed. Mild, reversible leukopenia and thrombopenia developed in 4 patients, and temporary hyperbilirubinemia developed in 1 patient with multiple liver metastases. No persistent changes in blood count or liver function occurred during a mean follow-up period of 43 mo.
During follow-up, CT scans revealed a partial response in 2 patients, stable disease in 9 patients, and disease progression in 5 patients, according to the guidelines of the Response Evaluation Criteria in Solid Tumors Group (6). In 1 patient, no follow-up was possible because of rapid disease progression.
DISCUSSION
131I-MIBG is an accepted treatment for neuroendocrine tumors, especially pheochromocytomas and neuroblastomas. Its crucial disadvantage, however, is that achievable tumor doses are mostly palliative. Tristam et al. (7) calculated a median tumor dose (DT) of 2.2 mGy/MBq (range, 0.4 < DT < 20 mGy/MBq). Several approaches to overcome this limitation have been proposed, including an increase in the injected activity (this approach is limited by bone marrow suppression, but the limitation may be overcome by using autologous stem cell support), an increase in specific activity (to increase tumor uptake), and use of non–carrier-added MIBG. Hoefnagel et al. (5) achieved an improved tumor-to-background ratio in 17 of 24 patients with carcinoid tumors by pretreatment with cold MIBG. The mean increase in uptake was 29%. Blake et al. found a 2-fold increase in tumor uptake after premedication with nifedipine in 1 of 5 patients (8).
We have chosen an alternative way to enhance tumor uptake that has rarely been described in the literature (9). Our approach, using intraarterial injection in the tumor-supplying artery, combined the advantages of targeted delivery (which is also used by other methods employing intraarterial delivery for tumor treatment, e.g., microspheres) with tumor-specific binding of MIBG and achieved a mean increase in tumor–to–whole-body ratio of 69%. This will lead to decreased bone marrow exposure and has the additional advantage of reducing injected activity and cost. This approach, however, is limited to patients with localized inoperable primary tumors or recurrences or with metastatic disease largely confined to the liver.
We did not aim to investigate any potential difference in MIBG kinetics between intravenous and intraarterial applications, such as potential shortening of the effective half-life. Whether intraarterial treatment produces a better response cannot be concluded from our results, because most patients had both intraarterial and intravenous treatments. The response rate achieved in our study is in line with previously published results (10–12), but further work is needed to establish the therapeutic effect of intraarterial versus intravenous treatment.
Intraarterial treatment is potentially associated with complications related to angiography, commonly including bleeding, vascular dissection, thrombosis, embolism, pseudoaneurysm, arteriovenous fistulas, and contrast reactions. For a transfemoral approach, these risks are in the order of 2%. Additionally, a temporary increase in hormone secretion after intraarterial application of 131I-MIBG may be caused either by higher tumor dose or by mechanical manipulation during angiography. However, we have found intraarterial 131I-MIBG therapy to be a safe alternative to conventional intravenous application.
As found in previous studies (6), a wide interindividual variability in tumor uptake was noted, and this variability applied to both intraarterial and intravenous treatments. Furthermore, the increase in tumor uptake achieved with intraarterial application was not significantly different between liver metastases and tumors located elsewhere.
CONCLUSION
Intraarterial treatment with 131I-MIBG has proven to be a safe alternative to standard intravenous application and provides a 69% higher mean tumor uptake. However, achievable enhancement varies widely between patients. We propose to attempt intraarterial MIBG treatment in every patient to assess its potential benefit.
Footnotes
Received Apr. 30, 2005; revision accepted Aug. 11, 2005.
For correspondence or reprints contact: Claudia Brogsitter, MD, Department of Nuclear Medicine, University of Dresden, Fetscherstrasse 74, 01307 Dresden, Germany.
E-mail: Claudia.Brogsitter{at}uniklinikum-dresden.de