Abstract
The purpose of this study was to assess the utility of dual-time-point imaging for identifying malignant lesions in the breast by 18F-FDG PET. Methods: Fifty-four breast cancer patients with 57 breast lesions underwent 2 sequential PET scans (dual-time-point imaging). The average percent change in standardized uptake values (SUVs) between time point 1 and time point 2 was calculated. All PET study results were correlated with follow-up surgical pathology results. Results: Of the 57 breast lesions, 39 were invasive carcinoma and 18 were postbiopsy inflammation. Among the invasive carcinoma lesions, 33 (85%) showed an increase and 6 (15%) showed either no change or a decrease in SUVs over time. The percent change in SUVs from time point 1 to time point 2 (mean ± SD) was +12.6% ± 11.4% (P = 0.003). Of the 18 inflammatory lesions, 3 (17%) showed an increase and 15 (83%) showed either no change or a decrease in SUVs. The percent change in SUVs from time point 1 to time point 2 (mean ± SD) was −10.2% ± 16.5% (P = 0.03). Of the 57 normal contralateral breasts, 2 (3.5%) showed an increase and 55 (96.5%) showed either no change or a decrease in SUVs. The percent change in SUVs from time point 1 to time point 2 (mean ± SD) was −15.8% ± 17% (P = 0.005). Conclusion: There is increasing uptake of 18F-FDG over time in breast malignancies, whereas the uptake of 18F-FDG in inflammatory lesions and normal breast tissues decreases over time. A percent change of +3.75 or more in SUVs over time is highly sensitive and specific in differentiating inflammatory lesions from malignant lesions.
PET with 18F-FDG has proven particularly helpful in oncology, in which its applications include initial diagnosis, staging, and therapeutic follow-up studies (1,2). Despite its proven utility, the application of PET is limited by its variable sensitivity and specificity estimates. One of the main reasons for this limitation is that many inflammatory lesions also have elevated 18F-FDG uptake in PET, leading to false-positive results (3,4). On the other hand, some types of cancers, for example, well-differentiated and lobular carcinomas of the breast and bronchoalveolar carcinomas, have abnormally low 18F-FDG uptake that is well below the diagnostic threshold for 18F-FDG uptake in malignant lesions (5,6). This situation leads to false-negative readings, which result in a lower sensitivity of PET in detecting true malignancies.
To better distinguish benign processes from malignant processes, researchers have made an important observation regarding 18F-FDG activity in PET. Most researchers quantify 18F-FDG uptake by calculating standardized uptake value (SUVs). However, when SUVs are measured on PET scans, there is a correlation between 18F-FDG uptake and time. Studies have shown that the uptake of 18F-FDG continues to increase in tumors for several hours after 18F-FDG injection (6–8). In fact, in many tumors, SUVs do not reach maximum until 130–500 min after 18F-FDG injection (7). This finding probably is related to the graded concentration of 18F-FDG in tumor cells, low glucose-6-phosphatase activity, and increased glucose uptake through glucose transporter proteins in these cells. In contrast, such a prolonged period of 18F-FDG uptake is rare in inflammatory lesions or normal tissues (4). Therefore, it has been deduced that this difference in the time course of 18F-FDG uptake could be used to improve the ability of PET to distinguish benign lesions from malignant lesions. Preliminary studies have been performed for PET of the head and neck and malignant lung lesions with dual-time-point imaging and have demonstrated a significant positive change in SUVs between the 2 time points (3,4,6,9).
In women, breast cancer has the highest incidence of all types of cancers and is the second leading cause of cancer deaths (10). 18F-FDG PET has proven to be quite effective in the management of breast cancer patients (11). In primary breast cancer diagnosis, PET has sensitivities of 63%–96% and specificities of 75%–100% (12–15). Although PET has been shown to have some potential for breast cancer diagnosis, the variable sensitivities and specificities continue to detract from its diagnostic utility. This finding is partially related to the lower metabolic activity of some types of breast cancers than of other malignancies. Consequently, single-time-point SUV analysis is a suboptimal method for assessing suspected breast cancer, and any method to improve the accuracy of PET in breast cancer characterization would be of value in improving the performance of this test. On the basis of the promising results from dual-time-point imaging research, the present study was undertaken to assess whether dual-time-point acquisition can improve the diagnostic utility of PET in breast lesions.
MATERIALS AND METHODS
Patient Population
Fifty-four patients (all women; age range, 32–77 y; mean age, 53 y) with 57 breast lesions were included in this prospective study. Patients for whom breast cancer was suggested by clinical examination and film mammography underwent diagnostic biopsy or excision biopsy. All patients underwent multimodality imaging techniques, such as MRI, ultrasonography, digital mammography, CT, and 18F-FDG PET, as a component of a National Institutes of Health–funded project for characterizing primary breast lesions and local–regional staging. Informed consent was obtained from all of the patients. None of the patients had received chemotherapy or radiation therapy before undergoing a PET scan. After 18F-FDG PET, all of the patients underwent surgical follow-up by either excision biopsy or mastectomy. Surgical pathology results were considered to provide the definitive diagnosis against which the PET study results were compared.
18F-FDG PET Imaging
All patients fasted for at least 4 h before the PET scan and had blood glucose levels of <140 mg/dL at the time of injection. PET was performed with a dedicated whole-body PET scanner (Allegro; Philips Medical Systems). Two scans were performed for all patients with an average time interval of approximately 38 min between the scans at the chest level. The first scan was performed as whole-body images from head to toe, with acquisition of 4 or 5 emission scans, resulting in a complete axial length of 64–76.8 cm. Scanning began at approximately 63 min after injection of 18F-FDG at 5.2 MBq (0.14 mCi) per kilogram of body weight. The second scan included the chest alone, with acquisition of 1 or 2 emission scans, resulting in a complete axial length of 25.6–38.4 cm. Scanning began at approximately 101 min after 18F-FDG injection. Transmission scans were performed for all patients to provide attenuation correction with a 137Cs point source. The patients did not have to leave the scanning table between the 2 scan acquisitions, minimizing patient motion artifacts. The ordered-subsets expectation maximization method was used to reconstruct all of the PET images (16).
Image Analysis
Two nuclear medicine physicians analyzed the data independently for this study. The SUVs were identical in 96% of cases with both of the observers. When there was a difference, a mean was calculated to determine the final SUV. After image reconstruction, a region of interest (ROI) measuring 12 × 12 mm (9 pixels) was carefully drawn on 4–6 PET scan slices at the site of the breast lesion. The slice with maximum 18F-FDG uptake in the ROI was chosen on the scan obtained at the first time point for quantitative measurement of the metabolic activity of the tracer (SUV). Next, an ROI of equal size was carefully placed at an equivalent site on the normal contralateral breast. Average SUVs were calculated for each ROI. From the ROIs, the SUVs were calculated with the following formula: mean ROI activity (MBq/g)/[injected dose (MBq)/body weight (g)].
The SUVs for imaging at the second time point were obtained with the same techniques as those used for imaging at the first time point. The percent change in the SUVs of a breast lesion between the 2 time points was calculated for the purpose of this investigation.
Statistical Analysis
All of the quantitative values were expressed in terms of mean ± SD. A paired t test was applied to determine the mean significant difference between time 1 and time 2, and an unpaired t test was applied to determine the mean significant difference between malignant lesions and inflammatory lesions. Receiver operating characteristic (ROC) analysis was performed to determine the best cutoff value for the percent change in average SUVs for malignant lesions. A P value of <0.05 was considered significant.
RESULTS
Of the 57 breast lesions in 54 patients, 39 were malignant and 18 were postbiopsy inflammation, with no residual tumor seen on surgical pathology analysis (i.e., the lesions were completely removed by the diagnostic or excision biopsy). Of the 39 malignant lesions, 31 were invasive ductal carcinoma, 6 showed features of both invasive ductal carcinoma and invasive lobular carcinoma, 1 was medullary carcinoma, and 1 was adenocarcinoma, as determined by histopathologic analysis.
Of the 39 malignant lesions, 33 (85%) showed an increase and 6 (15%) showed either no change (10%) or a decrease (5%) in average SUVs over time. All 6 lesions that showed either no change or a decrease in SUVs over time demonstrated a postbiopsy reaction. Table 1 shows the data for these 39 lesions. The average SUVs in the ROIs of these lesions (mean ± SD) were 2.88 ± 3.04 at the first time point and 3.38 ± 3.98 at the second time point. The calculated dual-time-point change for the average SUVs was +12.6% ± 11.4% (P = 0.003). If an SUV of 2.5 were considered the cutoff value for differentiating benign lesions from malignant lesions, then only 15 (38%) of the 39 lesions would have been considered malignant on the basis of this value.
Single- and Dual-Time-Point Imaging SUVs in Patients with Malignant Lesions
Of the 18 postbiopsy inflammatory lesions, 3 (17%) showed an increase and 15 (83%) showed either no change (11%) or a decrease (72%) in average SUVs over time. Of the 3 lesions that showed an increase in SUVs, 1 had subcutaneous infiltration of the 18F-FDG injection and chronic inflammation, 1 had proliferative fibroblasts, and 1 had a foreign-body giant-cell reaction. Table 2 shows the data for the patients who had increased 18F-FDG uptake attributable to inflammation but no residual malignant lesions after surgery. The average SUVs in the ROIs of these lesions (mean ± SD) were 1.29 ± 0.36 at the first time point and 1.19 ± 0.45 at the second time point. The calculateddual-time-point change for the average SUVs was −10.2% ± 16.5% (P = 0.03).
Single- and Dual-Time-Point Imaging SUVs in Patients with Postbiopsy Inflammation
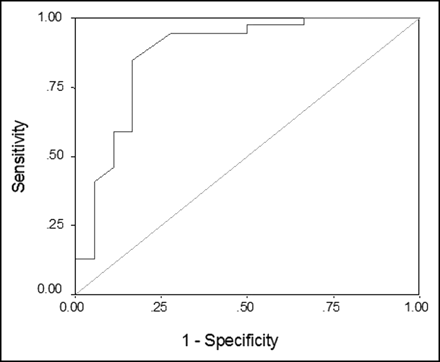
There was a significant difference between percent changes in average SUVs over time for malignant lesions versus inflammatory breast lesions (P < 0.0001). ROC analysis revealed that the ideal cutoff value for the percent change in average SUVs was 3.75, with a sensitivity of 82%, a specificity of 83%, and an area under the curve of 0.87 (Fig. 1). This sensitivity of dual-time-point SUV changes was significantly higher than that of the conventional single-time-point PET approach (82% vs. 39%) in differentiating malignant lesions from inflammatory lesions.
ROC curve for percent change in average SUVs.
Of the 57 contralateral normal breasts, 2 (3.5%) showed an increase and 55 (96.5%) showed either no change (22.5%) or a decrease (74%) in maximum SUVs over time. The average SUVs (mean ± SD) were 0.67 ± 0.24 at the first time point and 0.55 ± 0.21 at the second time point. The calculated dual-time-point change for the average SUVs was −15.8% ± 17% (P = 0.005).
There was a significant difference in percent change in average SUVs over time for malignant lesions versus normal breasts (P < 0.0001). However, there was no significant difference in percent change in average SUVs over time for inflammatory lesions versus normal breasts (P = 0.5).
DISCUSSION
The nonspecific increase in 18F-FDG uptake in benign inflammatory processes has been a source of false-positive results for PET. The single-time-point SUV of 2.5–3.8 has been cited as the optimal threshold in pulmonary malignancies for diagnosing lung cancer (17). Most inflammatory lesions would fall below this threshold, whereas the majority of malignant lesions would have high SUVs. However, this threshold may not be applicable to breast cancer, because the reported average SUV is lower in breast cancer cells as a result of less complete phosphorylation of 18F-FDG (18). In this study, 24 (62%) of 39 lesions had maximum SUVs of <2.5. If an SUV of 2.5 were considered the threshold for diagnosing malignancy, then many cancers would be categorized as benign. As with most other cancers, studies have shown that the majority of breast malignancies also show a gradual increase in SUVs over time after 18F-FDG injection (19). Dual-time-point analysis is likely to significantly improve both the sensitivity and the specificity of PET in diagnosing breast cancer, as in lung and head and neck malignancies.
Because the uptake of 18F-FDG in malignancies is expected to increase over several hours, the initial inclination would be to perform a single scan at a time point later than the usual 45–60 min. In theory, this strategy should lead to improved contrast between lesion and background and improved diagnostic accuracy, making dual-time-point scanning unnecessary. However, although this strategy may improve sensitivity, specificity may remain low because of the overall low uptake of these lesions. In the present study, even delayed average SUVs showed a minimal increase in sensitivity from 38% to 41%. Therefore, we believe that changes in dual-time-point SUVs would be a more valuable diagnostic tool than imaging at a delayed single time point alone. In fact, some studies with PET for pulmonary lesions showed that imaging at a delayed single time point failed to increase the sensitivity of diagnosis, and specificity actually decreased (6,9).
In a comparison of malignant tissues with contralateral normal breast tissues, there was a significant difference between changes in dual-time-point SUVs. Although malignant tissues showed positive dual-time-point SUV changes, normal breast tissues showed either no change or negative dual-time-point SUV changes. These data are supportive of the hypothesis for dual-time-point imaging for cancer diagnosis because it is expected that normal tissues will not accumulate 18F-FDG over an extended period of time. In some cases, normal breast tissues showed washout of 18F-FDG, and the SUVs actually decreased over time. These data are promising because they suggest that dual-time-point imaging would improve the sensitivity of the test, especially in patients with mammographically dense breasts, which show increased 18F-FDG uptake (20). Thus, the application of dual-time-point imaging in the breast would improve overall accuracy by correctly identifying benign normal tissues and inflammatory lesions, which at times may mimic cancer.
The second category of data in this study was for patients who had postbiopsy inflammation but no tumor detected by surgery. Despite the lack of malignant lesions, the PET scans demonstrated higher average SUVs in the abnormal breasts than in the normal breasts. At the first time point, for example, the mean maximum SUVs were 1.59 for the abnormal breasts and 0.79 for the normal breasts. This elevated 18F-FDG uptake likely was attributable to an inflammatory response from the diagnostic or excision biopsy performed before the PET study. Therefore, this category of lesions is representative of dual-time-point SUV changes in inflammatory lesions of the breast. Dual-time-point imaging showed either no change or a negative change in maximum SUVs in 83% of lesions. Interestingly, there was no significant difference between changes in dual-time-point SUVs for inflammatory sites and corresponding regions in the contralateral normal breast. Inflammatory lesions therefore are indistinguishable from normal breast tissues by dual-time-point analysis, as both show either no change or negative dual-time-point changes in SUVs most of the time.
Finally, it was also demonstrated that there was a significant difference between percent changes in average SUVs for malignant lesions versus inflammatory lesions (P < 0.0001). These data demonstrate that a percent change of +3.75 or more in SUVs over time allows sensitivity and specificity of more than 80% for distinction between inflammatory lesions and malignant lesions. The sensitivity of PET increased from 39% to 82% when the dual-time-point technique was used in place of single-time-point PET. Therefore, the change in SUVs over time estimated with the dual-time-point technique is helpful in differentiating breast cancer from inflammatory tissues.
On the basis of the above data, there was a trend for positive dual-time-point SUV changes in malignant lesions, whereas negative dual-point-time SUV changes were observed in inflammatory lesions and normal breast tissues. This study has some limitations. First, there was selection bias. Among the patients with benign findings, only 2 patients had benign lesions, whereas the remaining patients already had postexcision biopsy inflammation before PET. Second, all of these patients had had prior diagnostic or excision biopsies that likely removed a portion of the cancerous tissue. Consequently, the overall 18F-FDG uptake was decreased, lowering the contrast on the PET study. If PET had been performed as a screening modality for patients who had not undergone biopsies, then the dual-time-point SUV changes probably would have been clearer in this population. Finally, the time interval between the first and the second scans averaged 38 min. Given the initial low SUVs, breast cancer lesions may require a longer interval of time to allow adequate 18F-FDG accumulation. We suggest that future studies should extend the time interval between 2 scans to at least 60 min. In terms of practical applications, a 60-min time interval would be feasible for integration into clinical practice. Patients may be staggered between scans to maximize the time available and the number of scans performed. The use of dual-time-point imaging would add to diagnostic accuracy, especially for lesions with lower SUVs and in differentiating inflammation from malignant lesions; this increase in diagnostic accuracy would more than compensate for the extended length of each scan. Future research is needed to further elucidate the benefits of dual-time-point imaging in breast cancer.
CONCLUSION
There is an increase in the uptake of 18F-FDG over time in breast malignancies that is detected by dual-time-point PET. On the other hand, the uptake of 18F-FDG in inflammatory lesions and normal breast tissues decreases over time. A percent change of +3.75 or more in SUVs over time has high sensitivity and specificity in differentiating inflammatory lesions from malignant lesions.
Acknowledgments
This work was supported in part by Public Health Services Research Grant M01-RR00040 from the NIH. This work also was supported in part by the International Union Against Cancer, Geneva, Switzerland, under an ACSBI fellowship.
Footnotes
Received Jul. 12, 2005; revision accepted Aug. 11, 2005.
For correspondence or reprints contact: Abass Alavi, MD, Division of Nuclear Medicine, Hospital of the University of Pennsylvania, 110 Donner Bldg., 3400 Spruce St., Philadelphia, PA 19104.
E-mail: alavi{at}rad.upenn.edu