Abstract
Recombinant human thyroid-stimulating hormone (rhTSH) recently was introduced as a radioiodine administration adjunct that avoids levothyroxine (LT-4) withdrawal and resultant hypothyroidism. The pharmacokinetics of 131I after rhTSH administration are known to differ from those after LT-4 withdrawal but are largely nondelineated in the radioiodine therapy setting. We therefore sought to calculate the red marrow absorbed dose of high therapeutic activities of 131I given after rhTSH administration to patients with metastatic or inoperable locally recurrent differentiated thyroid cancer. We also sought to evaluate the clinical and laboratory effects of this therapy on the bone marrow. Methods: Fourteen consecutive patients received in total 17 131I treatments (7.4 GBq). Blood and urine samples were obtained at fixed intervals, and their activities were measured in a well counter. Based on blood activity, renal clearance of the activity, and residence times in red marrow and the remainder of the body, the red marrow absorbed dose was calculated using the MIRD schema. Additionally, we monitored for potential hematologic toxicity and compared platelet counts before and 3 mo after treatment. Results: The mean ± SD absorbed dose per unit of administered 131I in the red marrow was 0.16 ± 0.07 mGy/MBq. The corresponding total red marrow absorbed dose was 1.15 ± 0.52 Gy (range, 0.28–1.91 Gy). In none of the patients was hematologic toxicity observed. The mean ± SD platelet count (n = 13 treatments) was 243 ± 62 × 109/L before treatment and 233 ± 87 × 109/L 3 mo later, a slight and statistically insignificant decrease. After rhTSH-aided administration of high activities of 131I, the bone marrow absorbed dose remained under 2 Gy, the level long considered the safety threshold for all radioiodine therapy. Conclusion: Our specific findings imply that when clinically warranted, rhTSH should allow an increase in the therapeutic radioiodine activity. Such an increase might improve efficacy while preserving safety and tolerability; this possibility should be assessed in further studies.
Administration of high dosages of 131I is widely recommended as a treatment for metastatic and inoperable locally recurrent differentiated thyroid cancer (DTC) (1–3). In thyroidectomized patients, increased thyroid-stimulating hormone levels are necessary to maximize selective radioiodine uptake by neoplastic cells and traditionally have been obtained by withdrawing levothyroxine (L-T4) for 4–6 wk. However, L-T4 withdrawal typically induces symptoms of hypothyroidism that often physically and psychologically constrain active people for prolonged periods (4).
The availability of recombinant human thyroid-stimulating hormone (rhTSH) provides an alternative means to elevate the thyroid-stimulating hormone level without inducing hypothyroidism. In the United States and Europe, rhTSH is approved for use before thyroglobulin testing or diagnostic 131I scintigraphy in patients on L-T4 suppressive therapy. In addition, several reports have been published on the efficacy of rhTSH before administration of therapeutic activities of 131I (5–7).
As is the case with most radiopharmaceuticals, with radioiodine the bone marrow absorbed dose is one of the major factors limiting the size of the therapeutic activity that may be administered to a given patient. To calculate the bone marrow absorbed dose and, thus, the maximum tolerated activity for an individual, an understanding of the pharmacokinetics of large activities of 131I is necessary. Such pharmacokinetics are well characterized in patients whose LT-4 has been withdrawn and who have become hypothyroid (8,9). However, though it is known that the pharmacokinetics of 131I after rhTSH administration differ from those during hypothyroidism (10–12), these pharmacokinetics remain largely nondelineated with respect to large activities of radioiodine. To our knowledge, blood dosimetry of therapeutic activities of 131I has been reported only to a limited degree in 2 single-case reports (13,14). We therefore conducted the present study to calculate the red marrow and whole-blood absorbed doses of large activities of 131I given after administration of rhTSH. In addition, we sought to evaluate the effects of rhTSH-aided radioiodine treatment on the bone marrow by monitoring for potential related hematologic toxicity and by comparing baseline and posttreatment platelet counts.
MATERIALS AND METHODS
Patients
Patient characteristics are shown in Table 1. In total, 14 consecutive adult patients with metastatic (n = 12 patients, 13 treatments) or inoperable locally recurrent (n = 2 patients, 4 treatments) DTC were treated in 2 academic hospitals, 11 patients (treatments 1–14) at the University Medical Center in Utrecht, The Netherlands, and 3 (treatments 15–17) at the Ghent University Hospital, Belgium. Two patients were treated more than once (treatments 3, 6, and 10 and treatments 8 and 13), resulting in a total of 17 treatments for the series. Eight of 14 patients were female and 13 of 14, ≥64 y old.
Patient Characteristics
Before study entry, all patients had undergone total or near-total thyroidectomy followed by thyroid remnant ablation with a large activity of 131I. After ablation, 12 patients also had received one or more therapeutic activities of radioiodine after L-T4 withdrawal. Previous posttherapy scintigraphy showed pathologic 131I accumulation in metastatic or locally recurrent lesions in all patients. The study was approved by the local ethics committee, and all patients gave written informed consent to participate. Tumor dosimetry and response after rhTSH-aided radioiodine treatment in these patients have been reported elsewhere (7).
Treatment Protocol
Patients were instructed to follow a low-iodine diet for the 5 d before and 2 d after 131I administration. An intramuscular injection of rhTSH (0.9 mg) (Thyrogen; Genzyme Corp.) was given on 2 consecutive days, followed by oral administration of 7.4 GBq of 131I (Tyco Healthcare) on the third day. Treatment was given on an inpatient basis; patients were discharged 3 d after oral administration of the 131I, after the final collection of blood and urine samples.
Bone Marrow Dosimetry
Immediately before and at 1, 2, 4, 8, 24, 48, and 72 h after 131I administration, blood samples were drawn. In addition, urine was collected for the 72 h after radioiodine administration. The radioactivity of blood and urine samples and of a standard of known activity was measured in a well counter (Sample Changer type 5550; Packard Bioscience Co.). The blood data points were fitted to a biexponential time–activity-concentration function. For the second exponential function, a half-life of 80 d was used for 131I in thyroactive tissue. Use of this model recirculation of 131I is accounted for (15). It was assumed that the activity concentration in the red bone marrow could be estimated by the activity concentration in blood, using the patient-specific method of Sgouros (16,17), as the Na131I does not bind to marrow or bone. The circulating blood activity produces a red marrow dose, DRM, of:
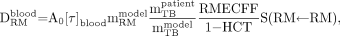 Eq. 1
Eq. 1
where A0 is the injected activity, [τ]blood is the residence time of the blood concentration (in h/L), mRMblood is the red marrow mass according to the bone marrow model (1.120 kg (18)), and mTBpatient and mTBmodel are the total body masses of the patient and according to the dosimetry model (MIRD: 73.7 kg (19)), respectively. The red marrow extracellular fluid fraction, RMECFF (=0.19 (16)), divided by 1 − HCT, the hematocrit (volume fraction of red blood cells), expresses the ratio of red marrow concentration to blood concentration, which usually lies between 0.2 and 0.4. The red marrow self-dose S-value, S(RM←RM), for 131I (1.55 × 105 mGy/MBq·s) was obtained from the RADAR (Radiation Dose Assessment Resource) Web site (20).
The whole-body activity equals the administered activity minus the excreted activity. These data points also were fitted to a biexponential time–activity function. Because the contribution of the activity in the bone marrow to the whole-body activity is small, the activity in the remainder of the body (Arem) was assumed to equal the whole-body activity: Arem(t) = Ah{a1 exp(−λ1,remt) + a2 exp(−λ2,remt)}, where Ah is the initial whole-body activity and λi,rem the whole-body clearance constants with relative contributions ai (for i = 1,2).
The dose to the red marrow from the whole-body distribution was estimated by the patient-specific method of Shen et al. (17):
 Eq. 2
Eq. 2
where τTB is the total-body residence time (=Ah/A0((a1/λ1,rem + λp) + (a2/λ2,rem + λp)), with λp the physical decay constant for 131I) and S(RM←TB) is the total-body–to–red-marrow S-value. Depending on whether bone metastases were observed, the total-body MIRD-based S-value was corrected for the bone component according to the method of Stabin et al. (21), yielding S(RM←TB) = 2.78 × 107 mGy/MBq·s without and S(RM←TB) = 4.04 × 107 mGy/MBq·s with uptake in the bone surfaces. Because no patients in this study showed evidence of diffuse uptake in the skeleton, only the first S-value was used for the radiation doses to the patients.
Whole-Blood Dose Calculations
The blood dose was calculated according to the method of Benua et al. (8). On the basis of the previously calculated cumulative blood activity, it was assumed for our patient population that 37 MBq/d/L of 131I delivered to the blood a β-dose of 10 cGy (assuming complete absorption) and a γ-dose of 20.4 cGy (assuming an absorption factor of 0.4).
Safety Assessment
Physician observation and patient reporting of adverse events were used to assess the safety and impact on the bone marrow of rhTSH-aided radioiodine treatment. Additionally, standard serum platelet counts were taken before and 3 mo after rhTSH-aided radioiodine treatment.
Statistical Analysis
Descriptive statistics were calculated for bone marrow, whole blood, and urinary dosimetry; urine excretion; and platelet count. All mean values are expressed as mean ± SD. Mean platelet counts before and after rhTSH-aided treatments were compared by a paired-sample t test using SPSS (version 11; SPSS Inc.) for Windows (Microsoft). A P value < 0.05 was considered significant.
RESULTS
The bone marrow and whole-blood dosimetry and urine and urinary 131I excretion in each patient are shown in Table 2. The calculated mean ± SD absorbed dose in the red bone marrow, expressed as dose per unit of administered 131I, was 0.16 ± 0.07 mGy/MBq for our patient group. The mean total bone marrow absorbed dose was 1.15 ± 0.52 Gy (median, 1.15 Gy; range, 0.28–1.91 Gy).
Bone Marrow and Whole-Blood Dosimetry and Residence Times
The mean whole-blood absorbed dose per unit of administered 131I was 0.23 ± 0.04 mGy/MBq. The mean total blood dose was 1.69 ± 0.34 Gy (median, 1.67 Gy; range, 1.09–2.27 cGy). Four patients had a whole-blood absorbed dose exceeding 200 cGy (treatments 2, 5, 9, and 12).
The mean residence time was 134 ± 60 h (median, 132 h; range, 17–231 h).
Pretreatment with rhTSH was very well tolerated. No serious or marrow-related side effects were noted. After 131I administration, a transient period of mild nausea was observed for 6 of 17 treatments. The nausea was treated with antiemetic drugs (8 mg of ondansetron) and resolved within 5 d in all cases. In addition, sialoadenitis of the parotid gland developed in 1 patient but quickly resolved after treatment with acetylsalicylic acid.
Pre- and posttreatment platelet counts were available for 13 treatments (10 patients). The mean platelet count before therapy was 243 ± 62 × 109/L. Three months after therapy, the mean platelet count slightly decreased to 233 ± 87 × 109/L, but this change was not statistically significant (P = 0.418). Over the 3-mo follow-up, platelet counts decreased after 8 treatments but increased after 5 (Table 3).
Platelet Counts
DISCUSSION
The bone marrow absorbed dose after treatment with large activities of 131I would be expected to be lower for patients given rhTSH, and therefore remaining euthyroid, than for patients subjected to L-T4 withdrawal and resultant hypothyroidism. The main explanation for this phenomenon lies in the lower renal clearance for hypothyroid patients than for euthyroid patients.
Changes in renal function are common during hypothyroidism, and an increased serum creatine and decreased glomerular filtration rate have been described (22). Decreased renal clearance would be expected to result in increased 131I retention, and indeed, Park et al. (10) reported that radioiodine retention after diagnostic 131I imaging was 50% greater in patients after withdrawal of L-T4 than in patients receiving rhTSH.
Another possible explanation for the differences in 131I pharmacokinetics between rhTSH administration and thyroid hormone withdrawal was offered by Menzel et al. (11) They recently demonstrated that rhTSH reduced the whole-body effective half-life of 131I after treatment with large activities, compared with observations for a separate cohort from whom thyroid hormone was withdrawn. Hypothyroid patients may have greater physiologic retention of radioiodine in the gastrointestinal tract (and presumably, other sites as well), as suggested by anecdotal impressions of greater background-to-noise ratios in posttherapy scintigrams taken after thyroid hormone withdrawal (11).
Our findings after 17 treatments given to 14 patients with metastatic or locally recurrent DTC suggest that the bone marrow absorbed dose indeed is lower after rhTSH-aided treatment than historically has been observed after thyroid hormone withdrawal–aided treatment. Although we calculated the red marrow absorbed dose using the MIRD schema, we also calculated the whole-blood radiation-absorbed dose by the approach of Benua et al. (8) to permit a comparison with historical data. We found a lower mean whole-blood dose per administered activity of 131I in our series than Benua et al. did in 59 patients with metastatic DTC receiving a total of 122 radioiodine treatments: 0.23 mGy/MBq versus 0.31 mGy/MBq (2.67 Gy; range, 0.45–7.40 Gy). Benua et al. observed serious complications such as nonfatal and fatal bone marrow depression, radiation pneumonitis, and vomiting persisting 30 d after 11% of treatments. Serious complications correlated with total blood radiation exceeding 2 Gy and with administration of >11 GBq of 131I per individual treatment. By contrast, our patients showed no severe radiation complications. Nausea and vomiting were mild and resolved within 5 d after treatment in all affected patients. In addition to the lower whole-blood absorbed dose, the superior safety profile seen in our series may be due to the activities that we administered. Although Benua et al. gave activities sometimes exceeding 11.1 GBq, we administered relatively conservative empiric activities of 7.4 GBq as standard treatment.
In therapy using radionuclides, a red marrow absorbed dose of 2 Gy is considered dose limiting (less than 5% damage in 5 y) (23). Our study showed that the bone marrow absorbed dose after rhTSH-aided administration of large activities of 131I fell below this threshold in all treatments. More important, in none of the patients was a clinically relevant bone marrow toxicity observed (only a slight, insignificant decrease in platelet counts after 3 mo). Blood tests were not performed within the 3-mo interval, and we therefore realize that a transient decline in platelet counts could have been missed. However, no intermediate-term (3 mo) hematologic side effects were observed.
In patients with a relatively high residence time (Table 2), a lower 131I clearance was observed; consequently, these patients had a higher red marrow dose delivered by whole-body activity. In our opinion, patients should be advised to drink at least 2 L of liquid per day to stimulate 131I clearance and thus decrease the whole-body exposure.
Our patient group had a mean red marrow absorbed dose of 0.16 mGy/MBq of administered 131I. This absorbed dose would allow a therapeutic activity of 131I higher than the current 7.4 GBq maximum that many centers allow for patients undergoing thyroid hormone withdrawal. This finding may have important therapeutic consequences, because a higher administered activity might well increase the tumor absorbed dose and thus therapeutic efficacy. Further studies are merited to test this hypothesis.
CONCLUSION
In the radioiodine therapy of DTC, a bone marrow absorbed dose of 2 Gy has long been considered the safety threshold that limits the amount of 131I activity that can be given. Our study showed that after rhTSH-aided administration of high activities of 131I, the bone marrow absorbed dose remained below the 2-Gy threshold in all patients and treatments. More important, no clinically relevant side effects were observed using administered activities of up to 7.4 GBq of 131I. Our specific dosimetric findings of a mean red marrow absorbed dose of 0.16 mGy/MBq per administered unit of 131I indicate that when clinically warranted, rhTSH should allow an increase in the therapeutic radioiodine activity. Such an increase might improve efficacy while preserving safety and tolerability; this possibility should be assessed in further studies.
Acknowledgments
This work was supported in part by Genzyme B.V. Netherlands, Naarden, The Netherlands. We are grateful to Rob Marlowe, Klaus Bacher, and Rudi Dierckx for their constructive critical reading and remarks.
Footnotes
Received Nov. 13, 2003; revision accepted Mar. 10, 2004.
For correspondence or reprints contact: Bart de Keizer, Department of Nuclear Medicine, University Medical Center Utrecht, Room E02.222, Heidelberglaan 100, 3584 CX Utrecht, The Netherlands.
E-mail: b.dekeizer{at}azu.nl







