Abstract
The evaluation of mediastinal lymph node involvement in non-small cell lung carcinoma (NSCLC) is very important for the selection of surgical candidates. PET using 18F-FDG has remarkably improved mediastinal staging in NSCLC. However, false 18F-FDG PET results remain a problem. This study was undertaken to identify histologic and immunohistochemical differences between cases showing false and true results of mediastinal lymph node involvement assessed by 18F-FDG PET. Methods: Preoperative 18F-FDG PET examinations were performed on 62 patients with NSCLC, and mediastinal lymph node sampling was done at thoracotomy or mediastinoscopy. In 111 lymph nodes, the size, glucose transporter 1 (Glut1) expression, grade of follicular hyperplasia, and involved proportion of tumor were examined and compared with the 18F-FDG PET findings. Results: Lymphoid follicular cells were strongly positive for the expression of Glut1. The grade of follicular hyperplasia in false-positive lymph nodes was higher than that in true-negative nodes (P < 0.001). The Glut1 expression of metastatic tumors was higher in true-positive nodes than that in false-negative nodes (P < 0.001). Metastatic squamous cell carcinomas showed stronger Glut1 expression than adenocarcinomas and no false-negative results on 18F-FDG PET. On the other hand, metastatic adenocarcinomas exhibited focal and weak Glut1 expression with frequent false-negative results. Conclusion: The results of this study indicate that (a) lymphoid follicular hyperplasia with Glut1 overexpression may have a causal relationship with high 18F-FDG uptake of false-positive nodes and (b) the lower expression of Glut1 in metastatic tumors, such as adenocarcinomas, might be responsible for false-negative lymph nodes.
Increased glucose uptake is one of the major metabolic changes found in malignant tumors (1), a process that is mediated by glucose transporters (Gluts) (2). On the basis of this relationship, PET using 18F-FDG has been widely used for the detection of primary and metastatic tumors in oncology patients (3). It is known that 18F-FDG PET is useful and superior to CT for the nodal staging of non-small cell lung carcinoma (NSCLC) (4,5). However, FDG is not a very tumor-specific substance, and benign lesions with increased glucose metabolism may give rise to false-positive (FP) results. Several studies have revealed that not only tumor cells but also fibrous tissue or inflammatory cells can accumulate FDG (6–8). These facts cause 18F-FDG PET to have a relatively low specificity (9). The FP results are one of the major problems in the clinical staging of lung cancer, especially nodal staging (Fig. 1). When contralateral mediastinal lymph node metastasis is suspected, surgical treatment is controversial despite the fact that surgery is the preferred treatment for NSCLC.
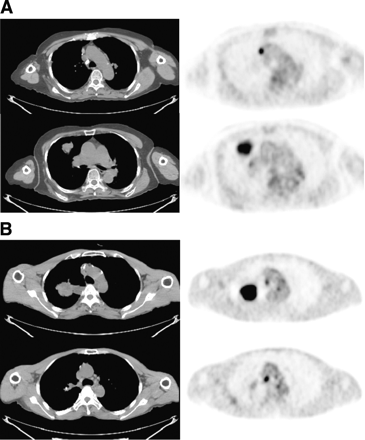
18F-FDG PET of mediastinal lymph nodes. (A) 18F-FDG PET image of TP lymph node in 64-y-old man. 18F-FDG PET scan shows hypermetabolic lung mass in right middle lobe with standardized uptake value (SUV) of 8.4 and small hypermetabolic lymph node in prevascular space with SUV of 6.3. Lymph node had high activity of 18F-FDG with focal appearance on 18F-FDG PET, so lesion was classified preoperatively as a metastatic lymph node. After surgery, adenocarcinoma and lymph node metastasis were proven on pathologic examination of surgical specimen. (B) 18F-FDG PET image of FP lymph node in 64-y-old man. 18F-FDG PET scan shows hypermetabolic lung mass in right upper lobe with SUV of 12.5 and small hypermetabolic lesion in right paratracheal lymph node with SUV of 4.3. Lymph node had high activity of 18F-FDG with focal appearance on 18F-FDG PET, so lesion was classified preoperatively as a metastatic lymph node. After surgery, squamous cell carcinoma was proven in the main mass, but there was no metastasis in lymph node.
The overexpression of Glut1 in human cancers is known to be closely related to 18F-FDG uptake in PET (10–12), though there is a controversial report (13). Several immunohistochemical studies have demonstrated overexpression of Glut1 in human malignancy and a correlation between Glut1 expression and neoplastic progression (14–17). However, Glut1 overexpression was also noted in cells with high glucose metabolism, such as the decidua of pregnant endometrium or the placenta (17). These findings intrigued us, and we hypothesized that Glut1 expression of lymph nodes might be correlated with 18F-FDG uptake. Any pathologic or physiologic condition showing Glut1 overexpression in lymph nodes could enhance 18F-FDG uptake. However, to our knowledge, no comparative study on the relationship between 18F-FDG PET results and Glut1 expression in mediastinal lymph nodes has been reported. This study was undertaken to compare the 18F-FDG PET results of mediastinal lymph nodes with histologic features and Glut1 expression.
MATERIALS AND METHODS
Subjects
Sixty-two patients with NSCLC (48 men, 14 women; age range, 39–77 y; mean, 62.5 y) who had surgery were enrolled in this study. Before being enrolled, all patients provided written informed consent for PET scanning and to have their tissues studied experimentally. The patients underwent preoperative 18F-FDG PET as a part of the staging work-up, and mediastinal lymph node sampling was performed at thoracotomy or mediastinoscopy.
CT examination was performed during preoperative work-up in all patients. The maximal diameter of enlarged lymph nodes, as determined from CT scans, was taken as an index of the size. The histologic diagnoses were 28 squamous cell carcinomas, 21 adenocarcinomas, 7 large cell carcinomas, 4 adenosquamous carcinomas, and 2 sarcomatoid carcinomas. One-hundred eleven mediastinal lymph nodes from 62 patients were selected for this study. Since the specific aim of this study was not to evaluate the sensitivity or specificity of PET, we selected all false results but only a portion of true results to serve as a control. The lymph nodes consisted of 4 groups: 41 true-positive (TP), 31 true-negative (TN), 27 FP, and 12 false-negative (FN) (Table 1).
Number of Studied Lymph Nodes by Histologic Subtype and Results of 18F-FDG PET
18F-FDG PET
18F-FDG PET was done 1–2 wk before surgery. All patients fasted for at least 6 h before PET imaging. Six patients had diabetes mellitus. In these patients, the dosage of insulin or oral hypoglycemic agent was reduced before scanning, and their blood glucose levels before scanning were <150 mg/dL. PET was performed using an Advance PET scanner (General Electric Medical Systems). Emission scans were obtained from the orbitomeatal line of the head to the thigh for 6 min per frame, 45 min after the intravenous injection of 370 MBq 18F-FDG. Transmission scans were performed for 3 min per frame using the 68Ge ring source and were used for the subsequent attenuation correction of the emission scans. Tomographic images were reconstructed with attenuation correction by an ordered-subsets expectation maximization iterative algorithm with a Hanning filter (cutoff frequency, 8.0 mm) and displayed as coronal, sagittal, and transaxial slices on a workstation. The standardized uptake value (SUV) was defined as the radioactivity concentration inside the region of interest divided by the injected activity per kilogram of body weight. Two nuclear physicians who were unaware of the CT and histologic results reviewed together and interpreted the PET images by consensus. Regional lymph nodes were considered positive for malignancy if focal prominent 18F-FDG uptake was found in 2 or more consecutive transaxial slices. Lymph nodes with an SUV of 3 or more were considered positive in case of failure of consensus as described (18). Each lymph node region visualized on PET was matched to a surgical specimen by the surgeon with expertise in mediastinal lymph node dissection.
Histologic Examinations of Lymph Nodes
Three serial sections of the lymph nodes were examined, and immunohistochemical staining for cytokeratin was performed to rule out micrometastasis in all negative lymph nodes. For histologically proven negative lymph nodes, Glut1 expression and the grade of lymphoid follicular hyperplasia were examined. Two independent pathologists graded lymphoid follicular hyperplasia by reviewing the same histologic sections without knowledge of the 18F-FDG PET results. Based on the area occupied by follicles in the lymph node, the grading of lymphoid follicular hyperplasia was assigned as follows: grade 1, <25%; grade 2, 25%–49%; grade 3, 50%–74%; and grade 4, 75%–100%.
For histologically positive lymph nodes, the proportion of tumor cells and Glut1 expression were determined. The proportion of tumor cells was determined as the relative tumor-occupying area in the cut surface of the involved lymph nodes.
Glut1 Immunostaining
One-hundred eleven lymph nodes from 62 patients were submitted for immunohistochemical study. Formalin-fixed, paraffin-embedded tissue sections were immunostained with rabbit anti-Glut1 polyclonal antibody (1:1,000; Chemicon). Four-micron sections were deparaffinized in xylene and rehydrated in a graded ethanol series. Endogenous peroxidase activity was blocked with 0.3% hydrogen peroxide in methanol. The sections were then washed with phosphate-buffered saline (PBS, pH 7.6), and microwaved in 10 mmol/L citrate buffer (pH 6.0) for 10 min to expose the antigens. After washing with PBS, the sections were incubated with bovine serum albumin for 10 min at room temperature to suppress nonspecific protein binding. Treated tissue sections were incubated with anti-Glut1 antibody at 37°C for 60 min and then rinsed with PBS. Sections were then incubated with biotinylated secondary antibody and streptavidin-biotin-peroxidase complexes (LSAB kit; DAKO). After washing, 3,3′-diaminobenzidine was used as a chromogen. Finally, the sections were counterstained with hematoxylin, dehydrated, and mounted. Adjacent sections incubated with rabbit IgG were used as negative controls. Red blood cells present in each section served as positive controls for Glut1. Glut1 expression was considered positive only if distinct membrane staining was present. Because the staining pattern is heterogeneous in the same tissue, we developed our own scoring method that includes staining intensity as well as the proportion of positive cells. Staining intensity was categorized as follows: 1, weak; 2, weak to medium; 3, medium; and 4, intense. The proportion of positive cells was graded on a semiquantitative scale as 1, 1%–9%; 2, 10%–24%; 3, 25%–49%; 4, 50%–74%; and 5, 75%–100%. Then the 2 values are multiplied and, finally, the Glut1 score is determined as follows: 1, ≤6; 2, ≤10; 3, ≤15; and 4, ≤20.
Statistical Analysis
The data are expressed as mean ± SD. Probability values of <0.05 indicated a statistically significant difference. Statistical analysis of size and tumor proportion between false and true results by PET was performed by the χ2 test. Statistical analysis of the grade of follicular hyperplasia and Glut1 score between false and true results by PET was tested by the Mann–Whitney nonparametric 2-sample test (SPSS version 10; SPSS Inc.).
RESULTS
Expression of Glut1
All metastatic tumors stained positively for anti-Glut1 antibody, and the staining patterns were heterogeneous by histologic type. The staining intensity and percentage of positive cells were considerably greater in the cell membranes of squamous cell carcinomas than that of adenocarcinomas or other NSCLCs (Fig. 2). Adenocarcinomas showed the most pronounced heterogeneity, with intracytoplasmic granular staining as well as weak membranous staining. All lymphoid follicular cells showed strong positivity along the cell membranes (Fig. 3).
Glut1 immunostaining in metastatic non-small cell carcinomas. (A) Squamous cell carcinoma with strong membranous Glut1 immunostaining (STAIN, ×200). (B) Adenocarcinoma with focal cytoplasmic Glut1 immunostaining (immunostain, ×200).
Glut1 immunostaining in lymphoid follicles. Note intense linear membranous staining in lymphoid follicular cells and erythrocytes as an internal positive control (immunostain, ×200).
Histologically Proven Negative Lymph Nodes
Glut1 expression was diffuse and uniform in the lymphoid follicles but was negative or faintly stained in the extrafollicular lymphoid cells. The grade of lymphoid follicular hyperplasia was 2.7 ± 1.1 in FP nodes and 1.6 ± 0.7 in TN nodes (P < 0.001) (Table 2). The Glut1 score was 3.0 ± 0.8 and 1.5 ± 0.9 in FP and TN nodes, respectively (P < 0.001). Maximal lymph node diameters estimated from CT scans were 11.0 ± 2.9 and 9.7 ± 2.8 mm for FP and TN nodes, respectively (P = 0.071).
Features of Histologically Proven Negative Lymph Nodes
Histologically Proven Positive Lymph Nodes
The Glut1 score of the metastatic tumor cells was 3.1 ± 0.9 and 1.8 ± 0.7 in TP and FN nodes, respectively (P < 0.001) (Table 3). The Glut1 score of the metastatic adenocarcinoma was 2.8 ± 0.6 and 1.5 ± 1.1 in TP and FN nodes, respectively (P = 0.015). The proportion of tumor cells in lymph nodes was 67.9% ± 35.0% (range, 5%–100%) and 52.5% ± 31.2% (range, 10%–90%) in TP and FN nodes, respectively (P = 0.095). The maximal lymph node diameters of TP and FN nodes were 13.3 ± 5.0 and 11.2 ± 2.7 mm, respectively (P = 0.584).
Features of Histologically Proven Positive Lymph Nodes
DISCUSSION
18F-FDG PET has been used for the nodal staging of NSCLC rather than CT because of its superiority at detecting metastatic lesions (4,5). However, FP results remain a problem, especially in Korea due to frequent upper respiratory infection histories of the patients. This remarkable high rate of FP results in PET might be due to the high prevalence of tuberculosis in Korea (19).
18F-FDG uptake is known to be closely related to the overexpression of Glut1 in human cancers, including lung cancer (10–12). This fact prompted us to hypothesize that the elevated 18F-FDG uptake of negative lymph nodes would be correlated with Glut1 expression of lymphocytes. The overexpression of Glut1 could enhance the uptake of glucose into mediastinal lymph nodes, and this increased glucose influx might contribute to the FP results of 18F-FDG PET.
Our main interest was to determine whether Glut1 overexpression is strictly related to metastatic tumors in mediastinal lymph nodes. To answer this question, we studied the expression of Glut1 in metastatic and nonmetastatic lymph nodes. There was no difference in the Glut1 score between TP and FP lymph nodes (Tables 2 and 3). In addition, we examined the grade of follicular hyperplasia and tumor volume to determine whether these factors are associated with 18F-FDG uptake. Our study showed that the grade of follicular hyperplasia in mediastinal lymph nodes is significantly higher in FP nodes than that in TN nodes. This can be explained by the finding that follicular cells consistently express Glut1 protein. A lymphoid follicle consists of metabolically active cells and the proliferation rate of follicular hyperplasia is higher than that of follicular lymphoma (20). A positive correlation between 18F-FDG uptake and cellular proliferation has been reported in breast cancer (21). Therefore, it might be assumed that the more metabolically active a cell, the higher its 18F-FDG uptake. As reported in previous studies, inflammatory processes may also result in increased 18F-FDG uptake (6–8,22,23). These findings indicate that increased 18F-FDG uptake may not be entirely specific for malignant tissue. Lymphoid follicular hyperplasia is a type of chronic lymphadenopathy. Patients with long histories of smoking, chronic airway disease, or tuberculosis may have mediastinal lymphoid follicular hyperplasia and FP results on 18F-FDG PET. In this study, FP results appeared to be more frequent in patients with squamous cell carcinomas than in those with adenocarcinomas. We presume that there might be a positive correlation between heavy smoking and the FP rate on 18F-FDG PET. A detailed clinicopathologic correlative study is warranted to confirm this presumption. No FN nodes were found in squamous cell carcinomas but frequent FN nodes were found in adenocarcinomas. Glut1 immunostaining was stronger and more diffuse in squamous cell carcinomas than that in adenocarcinomas. Moreover, Glut1 expression tended to be localized on the cell membranes in squamous cell carcinomas, which may reflect higher transport activity than that in adenocarcinomas with predominant cytoplasmic staining. These results correspond with earlier reports that described higher 18F-FDG uptake and stronger Glut1 expression in squamous cell carcinomas than that in adenocarcinomas of the lung (12,24,25). Comparing the FN and TP lymph nodes involved by adenocarcinomas, the tumor volume and size did not differ (P = 0.213 and P = 0.852, respectively) but the Glut1 score was significantly different (P = 0.015). These results suggest that lower Glut1 expression in some adenocarcinomas might be responsible for FN results. The constellation of 18F-FDG uptake in mediastinal lymph nodes and histologic subtypes of NSCLC would improve the accuracy of 18F-FDG PET interpretation.
CONCLUSION
The expression of Glut1 in mediastinal lymph nodes is significantly higher in FP nodes than that in TN nodes, and the grade of lymphoid follicular hyperplasia is a major pathologic factor that influences 18F-FDG uptake in mediastinal lymph nodes. The Glut1 expression of metastatic tumors was higher in TP nodes than that in FN nodes. These data indicate that lymphoid follicular hyperplasia with Glut1 overexpression may have a causal relationship with high 18F-FDG uptake of FP nodes, and the lower expression of Glut1 in metastatic tumors, such as adenocarcinomas, might be responsible for FN lymph nodes.
Acknowledgments
This work was supported by Korea Cancer Center Hospital grant 50202-2001. The authors appreciate the critical review and comments on this manuscript by Ung-Gu Kang, MD.
Footnotes
Received Jul. 1, 2003; revision accepted Jan. 14, 2004.
For correspondence or reprints contact: Sang Moo Lim, MD, Department of Nuclear Medicine, Korea Cancer Center Hospital, 215-4, Gongneung-Dong, Nowon Gu, Seoul, 139-706, Korea.
E-mail: smlim328{at}kcch.re.kr