Abstract
PET/CT is a new imaging technology that has already found a number of clinical applications in oncologic imaging. Widespread introduction into clinical practice started approximately 2.5 years ago. Consequently, the available data are largely preliminary. Nevertheless, it can already be stated that the synthesis of structural and metabolic information improves the accuracy of primary staging and the detection of recurrent disease and has the realistic potential to change patient management in 10 to 20% of cases. PET/CT fusion images can directly guide biopsies or surgical interventions. This article summarizes preliminary data of PET/CT studies and highlights potential clinical applications for PET/CT, with particular emphasis on lymphoma, melanoma and gastrointestinal tumors.
In a recent review, we described our experience with the technical aspects and performance of PET/CT, an emerging imaging technology in nuclear medicine (1). The interested reader is referred to this article for a general discussion of PET/CT technical performance, prominent artifacts, and image interpretation, including benefits in the head and neck and abdomen, especially in differentiating benign and malignant tumors, in comparison with dedicated PET alone. In the following article, we focus on specific clinical applications and discuss how PET/CT could be integrated into the management of certain common human tumors, including lymphomas, melanomas, and gastrointestinal malignancies.
LYMPHOMA
18F-FDG PET is now a widely accepted imaging modality in the initial staging and posttherapy follow-up for patients with both Hodgkin’s disease and non-Hodgkin’s lymphoma (2–5). Where available, it has largely replaced the gallium scan as the nuclear medicine imaging modality of choice for this group of patients. For the initial staging of patients, it has been shown that 18F-FDG PET is superior to the gallium scan in detection of lesions, especially in the abdomen and extranodal sites (6,7). For posttherapy follow-up, it is helpful in characterizing residual mass lesions as either fibrosis or active lymphoma. Preliminary evidence also suggests that 18F-FDG PET is useful as a prognostic indicator, using either the PET scan after chemotherapy or the initial PET with standardized uptake value (SUV) measurements (6,8–10).
The advantage of 18F-FDG PET when compared with conventional imaging modalities lies in the high tumor-to-background ratio, resulting in high sensitivity and specificity for lymphoma. However, because of the relatively poor visualization of normal organs and tissues on PET images, lesion localization is suboptimal. This is particularly problematic for non-Hodgkin’s lymphoma, in which disease is frequently extranodal and can occur in almost any site and organ in the body. The lesion location is usually obvious when reading the PET side by side with a CT scan, but fusion images from PET/CT provide significantly increased confidence in characterizing the abnormality and locating the lesion (Fig. 1). Occasionally, a PET abnormality does not correlate with any obvious CT findings. In this situation, the PET/CT would be helpful in localizing the lesion accurately on CT images and, by doing so, would provide valuable information about the true nature of the 18F-FDG uptake (Fig. 2). For patients in whom biopsy is required, PET/CT fusion images are valuable in determining biopsy sites (Fig. 3).
Patient (65-y-old man) with diffuse large-cell lymphoma referred for follow-up evaluation. PET showed focal 18F-FDG activity in anterior abdomen, which could be result of normal bowel activity or disease. The transaxial CT (A), PET (B), and fusion images (C) showed 18F-FDG uptake localized to mesenteric nodules seen on CT, confirming tumor activity.
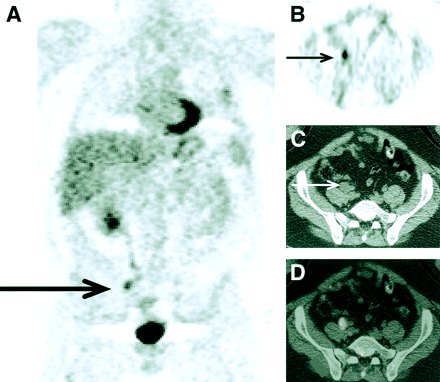
Patient with lymphoma, referred for staging. (A) PET images showed intense 18F-FDG uptake over left sacral region. (B) A corresponding CT image showed no definite lesion. (C) PET/CT fusion image unequivocally localized abnormal 18F-FDG uptake in left sacral ala, resulting in change in staging and patient management.
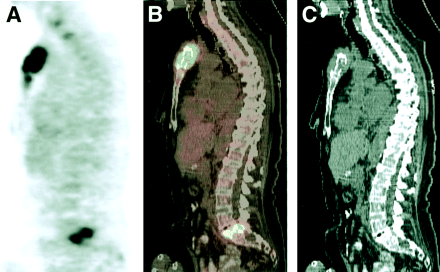
Patient with diffuse large B-cell lymphoma and widespread osseous involvement on 18F-FDG PET, but the posterior iliac crest biopsy was normal. Sagittal PET (A), CT (B), and fusion images (C) showed abnormal 18F-FDG uptake in sternum and adjacent soft tissue. Subsequent biopsy confirmed lymphomatous involvement.
Transformed low-grade lymphoma presents a unique situation in which PET/CT fusion imaging may be crucial in arriving at a diagnosis. SUVs are usually in the low-to-medium range in low-grade lymphoma (11). An unexpected sudden increase in 18F-FDG uptake in part or all of the known disease sites during follow-up suggests transformation to a higher grade tumor. Definitive diagnosis would require tissue biopsy, usually directed to the site that is enlarging in size on CT. The PET scan provides additional information for identifying the location of the transformation for biopsy, because sometimes only part of an enlarging lesion has transformed.
PET can be misleading when 18F-FDG uptake is increased in normal tissues, such as the intrinsic laryngeal muscle, especially when the anatomy or physiology is distorted after surgery or in a patient with unilateral vocal cord paralysis. 18F-FDG uptake in inflammatory lesions is a well-known cause of false-positive findings on PET. 18F-FDG uptake in brown fat in the neck and supraclavicular area, a normal variant, is less well known and can mimic extensive lymphadenopathy (Fig. 4). In fact, the phenomenon of 18F-FDG uptake in brown fat was first discovered when PET/CT fusion images showed 18F-FDG concentration in the adipose tissue rather than in muscle or lymph node as previously assumed (12). When 18F-FDG uptake in brown fat and lymphoma coexist, PET/CT fusion images are essential in diagnosis or exclusion of 18F-FDG–avid lymphadenopathy in the head and neck (1).
Patient (11-y-old girl) with Hodgkin’s disease imaged for posttherapy follow-up. She was clinically disease free. (B) 18F-FDG PET scan showed focal increased uptake in bilateral supraclavicular regions, axillae, and lower rib cage laterally, suggestive of widespread lymphadenopathy. (A) However, on CT images, no abnormal lymph nodes or mass lesions were seen in those areas. (C) Foci of increased 18F-FDG uptake localized to adipose tissue on PET/CT fusion images.
MALIGNANT MELANOMA
Melanoma staging is now based on the following factors: primary tumor thickness best predicts survival (1); ulceration upstages patients in each T stage subgroup (2); the number of lymph nodes in N staging is significant (3); clinical versus pathologic staging incorporates lymphatic mapping data and pathologic assessment of micrometastatic disease within lymph nodes (4); and stage IV metastatic disease is subclassified based on anatomic site and elevated serum lactate dehydrogenase (5).
18F-FDG PET is of limited use in patients with early-stage disease without nodal or distant metastases (stage I–II), because sentinel node biopsy is much more sensitive in detecting microscopic lymph node metastases (13). However, PET has proven useful in the detection of distant metastases. In a prospective study of 106 scans in 95 patients with stage III regional disease (with lymph node metastases), Tyler et al. (14) reported a sensitivity of 87% and a positive predictive value of up to 90% when pertinent clinical information was available to exclude some of the false-positive findings. More important, they showed that unexpected findings seen on PET resulted in a change in management in 15% of cases, including the detection of distant metastases (stage IV).
Malignant melanoma is well known for its propensity to spread to unusual sites, including the gastrointestinal tract, the myocardium, and the leptomeninges. Because of the high tumor-to-background ratio, 18F-FDG PET can highlight metastases at unusual sites that are easily missed with conventional imaging modalities. For the same reason, there is difficulty in locating PET abnormalities when a contemporaneous CT shows no abnormality in the same area. The PET/CT scan enables the precise localization of the PET abnormality. A decision on further imaging, biopsy, or therapy can then be made, as shown in Figures 5–7.
Patient with malignant melanoma and suggestive left lung lesion, referred for evaluation. He also complained of lower back pain, for which lumbar spine MRI was performed and reported negative. (A) Transaxial PET image shows irregular intense 18F-FDG uptake in mid-thoracic spine. (B) Corresponding CT image shows increased paravertebral soft tissue. (C) PET/CT fusion images demonstrate metastasis in right paravertebral region with extension through neural foramen into spinal canal. (D) This was subsequently confirmed on MRI, and patient underwent laminectomy.
48 year old man with malignant melanoma, referred for follow up evaluation when CT discovered two suspicious lesions in the spleen. (A) PET images show focal FDG uptake in the left mid abdomen. On the CT (B) and fusion images (C) the lesion could be localized to the third portion of the duodenum. Even in retrospect this lesion could not be identified in the diagnostic CT with contrast. This lesion was later also visualized with a small bowel series and at surgery confirmed to be metastatic melanoma.
Patient (34-y-old man) with malignant melanoma of the back, status postexcision, referred for evaluation of possible recurrence in left axilla. PET images demonstrated intense focal 18F-FDG uptake in left axilla (not shown), as well as focal uptake in right upper quadrant (A, transaxial PET image). (B) This was shown in PET/CT fusion image (lower panel) to be in gallbladder, later confirmed by sonogram and CT.
GASTROINTESTINAL MALIGNANCIES
Only limited data are available on the role of PET/CT in patients with gastrointestinal malignancies. The first published study reported that the staging accuracy increased from 78% with PET alone to 89% with PET/CT (15). Because of limited published data, the following discussion is largely based on preliminary data that have been presented in abstract form and on our own experience using PET/CT imaging in more than 9,000 patients with cancer.
Patient Preparation and Potential Pitfalls
A minimum of 450 mL oral contrast (e.g., 2.1% barium sulfate) is administered at 60–90 min before imaging. This will opacify the small intestine and, to a varying degree, the colon. Immediately before imaging, the patient is asked to drink another 50–75 mL oral contrast, which opacifies the stomach and duodenum. As an alternative to oral contrast for imaging the upper abdomen, patients may be asked to drink approximately 1,500 mL water (“hydro-CT”) shortly before the examination. In selected patients, it may be advantageous to distend the stomach to better evaluate wall thickening. This can be done by having the patient swallow CO2-producing granules or a larger amount of water just before imaging.
Potential misalignment between CT and PET datasets can occur in imaging the upper abdomen as a result of respiratory diaphragmatic motion. Major misalignments can usually be avoided if the CT is acquired during shallow breathing or in midexpiration. Major misalignments are observed when the CT is acquired during maximum inspiration, but these are easily recognized. Care must be taken to avoid misinterpretation related to respiratory motion. Depending on the breathing cycle during CT acquisition, hypermetabolic lesions located in the upper abdomen can be displaced and, on fusion images, appear to be located in the lung bases or heart (Fig. 8). Once CT and PET images are reviewed independently, such misalignment is again easily recognized (1).
Patient with recurrent bladder cancer, referred for restaging. (A) Coronal CT, PET, and fusion images showed focal 18F-FDG uptake, apparently located in heart (red cross). (B–D) The corresponding transaxial images again showed abnormal 18F-FDG uptake in this location. (E, F) CT images (“liver window”) showed hypodense lesions (metastases) in liver. Apparent abnormal 18F-FDG uptake in the heart was the result of misalignment between CT and PET images, related to deep inspiration during CT image acquisition.
Overview of Clinical Applications
The ability of 18F-FDG PET to detect malignant lesions in the gastrointestinal tract is affected by three major factors: size of the lesion, intensity of tracer uptake (lower detection rate of only 40%–60% for mucinous tumors [16,17]), and location of the lesion. Because PET/CT combines structural and metabolic information, it has the ability to overcome at least some of these potential limitations. Smaller lesions or those with relatively low 18F-FDG uptake will be identified and classified accurately because of the additional anatomic information provided by the CT component of the study (Fig. 9). In addition, PET/CT fusion enables the reader to differentiate between several physiologic variants and true abnormalities, particularly in the case of focal tracer accumulation (Fig. 10).
Patient with seminoma, status postorchiectomy, retroperitoneal lymphadenectomy, and radiation therapy to retroperitoneum. He presented with rising β-human chorionic gonadotropin tumor marker levels. (A, B) The sagittal and transaxial PET images showed focal 18F-FDG uptake in retroperitoneum. (C) Noncontrast CT showed no clear abnormality. (D) The fusion image localized abnormal 18F-FDG uptake to the small retroperitoneal lymph node, later proven to be metastatic disease.
Patient with Non-Hodgkin’s lymphoma in the left chest wall, status post chemo- and radiation therapy, study is done for restaging. (A, B) Coronal and transaxial PET shows focal abnormal 18F-FDG uptake in the right lower pelvis, uncertain whether this represents excreted urine or nodal disease. (C) CT shows enlarged right external iliac node, and fusion image (D) localizes the abnormal 18F-FDG uptake to this node.
In a retrospective analysis of our initial experience with PET/CT in 68 patients with abdominopelvic malignancies, we noted improvement in anatomic localization for 64% (73/113) of lesions and a decrease in the number of equivocal readings by more than 60% (18). Findings that were considered equivocal by PET alone could be classified as clearly benign, including intestinal and ureteral activity and posttraumatic or postsurgical changes. At the same time, 13 malignant lesions that previously had been considered equivocal by PET alone were newly identified. Newly identified lesions included nodal metastases, a cervical cancer, a case of intestinal lymphoma, and a case of recurrent colorectal carcinoma. In almost all instances, this was related to the additional anatomic information provided by the CT component of the study. Hence, in general, PET/CT improves the anatomic localization of PET abnormalities and improves the certainty of PET image interpretation. In many cases, this results in a change in patient management. Examples will be presented by disease entity.
PET/CT is also uniquely suited for radiation therapy planning. Previous studies in patients with lung, head-and-neck, and rectal cancer (19,20) have shown that PET leads to an adjustment of target volumes in many patients and that treatment based on PET plus CT (rather than CT alone) improves patient outcomes (21). There is little doubt that the same holds true for most other malignancies that are 18F-FDG avid.
Esophageal and Gastric Carcinoma
CT of the chest and abdomen is standard in the evaluation of patients with esophageal and gastric carcinomas. However, despite marked improvements with the implementation of thin-section multislice techniques, the accuracy of CT is still relatively low. For example, CT accuracy for T staging alone in esophageal cancer varies between 40% and 60%, and for N staging it ranges from 39% to 74%. In gastric cancer, the accuracy for nodal metastases is approximately 60%–65%. Overall, approximately one third of patients with esophageal and gastric carcinomas who undergo surgery are found to have occult metastases. Definitive staging, therefore, is possible only intraoperatively and in the histologic specimen. Nevertheless, CT can provide valuable information on wall thickness, assessment of direct transmural invasion of the tumor into the mediastinum or perigastric fat tissue, and the presence of regional lymphadenopathy and distant metastases in lung, liver, adrenal glands, or distant nodes. CT can demonstrate tumor invasion into adjacent organs in the mediastinum (e.g., tracheobronchial, encasement of aorta) or upper abdomen as well as some suggestion about the presence of peritoneal carcinomatosis. Most of all, CT imaging contributes to treatment decisions, because it provides information about the presence and extent of transmural spread and invasion into adjacent fat tissue, thereby assisting in the decision to perform curative surgery or select other (palliative) treatment approaches.
Although PET detects almost all esophageal primary tumors, the detection rate for gastric cancers depends on the histologic subtype (lower sensitivity for intestinal-type tumors and for diffusely growing tumors, such as Borrmann III and IV tumors [17]). For both diseases, PET and CT have limited sensitivity for the detection of regional nodal metastases. With 18F-FDG PET, this is probably the result of intense tracer uptake in most primary tumors and a resulting inability to distinguish focal uptake in adjacent nodes. PET/CT fusion imaging has the potential to improve the specificity of nodal staging by CT alone by identifying metastatic deposits in nodes that show nonspecific enlargement or are of borderline size.
PET is more accurate than CT for the detection of distant metastases. This is important, because regional adenopathy immediately adjacent to the esophagus or stomach can be resected and does not preclude curative surgery, whereas distant metastases (liver, lung, supraclavicular lymph nodes, intraperitoneal spread, bone) are contraindications for radical surgery.
With PET/CT fusion imaging, the functional advantages of PET and the structural advantages of CT combine to enhance the detection rate for metastasis from 80% to 90% accuracy. If the tumor is anatomically evident but metabolically inactive (17), it will be detected by CT. If the tumor shows increased glycolysis but no CT abnormalities, it will be detected by PET (Fig. 11). In a recent study of patients with esophageal cancer (initial staging, evaluation of neoadjuvant chemotherapy, or postsurgical follow-up), PET/CT appeared superior to PET or CT alone and had an effect on further management in 22% of patients (22). Management changes were related to better localizing PET abnormalities, retrospectively detecting true abnormalities on concurrent contrast CT, guiding endoscopy to the site of suspicious lesions, and eliminating the need for further work-up that would have been necessary with CT or PET findings alone.
Patient with esophageal carcinoma, referred for initial staging. (A) The sagittal PET image showed abnormal 18F-FDG uptake in primary tumor and lower T-spine. (B) A corresponding bone scan showed mildly increased tracer uptake in lower T-spine, inconclusive. (C) A transaxial PET image again showed abnormal 18F-FDG uptake in primary and in spine, which were clearly localized on fusion images (D). (E) CT image showed only subtle abnormality in this vertebra.
Colorectal Cancer
The diagnosis of colorectal carcinoma is generally based on colonoscopy and biopsy. Imaging studies (CT virtual colonoscopy, 18F-FDG PET, or PET/CT) can be used for screening purposes in selected groups of patients at risk for colon carcinoma or otherwise unexplained elevated serum levels of carcinoembryonic antigen (CEA).
The role of imaging studies in primary staging is generally limited because of lack of accuracy for T and N stage and because most patients will benefit from tumor resection to avoid intestinal obstruction or bleeding. Endoluminal ultrasound seems to have the highest accuracy for assessment of tumor infiltration of the bowel wall and adjacent fat tissue, but the depth of bowel wall infiltration and spread to regional lymph nodes is frequently obtained only intraoperatively or histopathologically. Nevertheless, an attempt to detect nodal or organ metastases is important in planning the general therapeutic approach (i.e., palliation versus curative tumor resection).
Recurrent Disease
Early detection of recurrent disease leads to improved re-resection and survival (23). Local recurrent disease in the pelvis is often difficult to diagnose with CT or MRI. This is largely because of the frequent presence of nonspecific fibrotic/scar tissue after surgical interventions or radiation therapy. PET alone is clearly superior to CT in this setting, with a reported accuracy of 95% as compared with 65% for CT (24). However, despite good sensitivity of 97%, the specificity of 18F-FDG PET averaged only 76% in a recent metaanalysis (25), reflecting a greater number of false-positive findings. This is at least in part related to the lack of anatomic information. PET alone is therefore insufficient in guiding biopsies or therapy.
PET/CT has the potential to further improve accuracy in detecting and staging recurrent disease. In a recent study of 21 patients with colorectal carcinoma, PET/CT improved anatomic localization of PET abnormalities and increased certainty in reporting PET findings as clearly normal or clearly abnormal/malignant by 13% (reviewer 1) and 22% (reviewer 2) (26). Berger et al. (27) studied 65 patients with known or suspected recurrent colorectal carcinoma, using histopathology or clinical follow-up as standards of reference. They reported less over- and understaging with PET/CT compared with PET alone (10% vs. 31%). Sensitivity and specificity for local recurrence were 96% and 97%, respectively, with PET/CT, compared with 77% and 89%, respectively, with PET alone. Similarly, the sensitivity and specificity were higher for the detection of metastases with PET/CT (95% and 98%, respectively) than with PET alone (66% and 79%, respectively). Because of the added anatomic information, PET/CT also improved interobserver agreement.
Overall, we believe that PET/CT fusion imaging should be the preferred imaging modality in these patients, because it identifies and localizes the disease in one setting and can guide diagnostic or therapeutic interventions (Fig. 12).
Patient with rising CEA, status post resection of rectal carcinoma. (A) Coronal PET image showed focal 18F-FDG accumulation in left lower pelvis, of uncertain significance. (B) CT showed nonspecific soft tissue along left pelvic side wall. (C) The fusion image clearly showed focal abnormal 18F-FDG uptake within this soft tissue. (D) PET/CT guided biopsy proved recurrent rectal carcinoma in this location.
Liver Metastases
Early identification of liver metastases provides the opportunity for neoadjuvant chemotherapy and resection, which may prolong survival, particularly for patients with colorectal carcinoma (28,29). In many institutions, contrast-enhanced spiral CT is the primary imaging modality for the detection, localization, and characterization of focal liver lesions (30), and dual-phase CT has become the standard technique for the evaluation of equivocal liver lesions. CT portography is more sensitive than regular CT with intravenous contrast but may generate false-positive findings (31). The potential role of various MR techniques and new MR contrast agents is under investigation (32,33). In a recent metaanalysis, 18F-FDG PET appeared more sensitive than ultrasound, CT, or MRI for the detection of liver metastases (34). Larger studies comparing 18F-FDG PET or PET/CT fusion with newer CT or MRI techniques are lacking. However, because PET alone is already more accurate than CT and fusion imaging helps to localize abnormalities, it is possible to foresee an immediate impact on patient management. PET/CT fusion imaging may be of particular value in patients with several hypodense liver lesions that are not clearly characterized by CT alone (Fig. 13) and in patients in whom regular contrast CT (or even portal-venous angio-CT) fails to detect metastases in the setting of a rising CEA (Fig. 14). In these cases, PET/CT has the ability to directly affect patient management by guiding biopsies or directing surgical resections of liver lesions.
Patient with colon cancer, presurgical staging. (A, B) CT showed large hypodense liver lesions that were interpreted as benign cysts. (C) The fusion image again showed large liver cyst but also abnormal 18F-FDG uptake medially to one of liver cysts, without corresponding abnormality on this noncontrast CT or subsequent CT with intravenous contrast (images not shown). The lesion was proven to be metastasis.
Patient with colorectal carcinoma, status postresection, presenting with rising CEA tumor marker levels. (A) CT with intravenous contrast did not show metastatic disease. (B) Transaxial PET showed focal abnormal 18F-FDG uptake in right lobe of liver, which could be clearly localized on fusion image (C). Biopsy proved liver metastasis.
Monitoring of Neoadjuvant Therapy
Neoadjuvant chemo- or radiation therapy is performed in patients with rectal cancer with the aim of achieving local downstaging of the primary tumor in patients with otherwise unresectable disease (uT4) and to eradicate disseminated tumor cells not detected by current staging methods, thereby decreasing disease relapse and improving patient survival. PET/CT fusion imaging is not necessary to monitor the results of neoadjuvant therapy, because the response is exclusively judged by changes in the intensity of 18F-FDG uptake in the primary. A comparison of regular PET images from the pre- and posttreatment settings is therefore sufficient in this group of patients.
Other Potential Applications of PET/CT in Colorectal Disease
Certain patterns of intestinal 18F-FDG uptake correspond to various pathologic features (35). In particular, focal intestinal 18F-FDG uptake is highly suggestive of the presence of premalignant or malignant bowel lesions (36). With the advent of PET/CT, it is now possible to exactly localize these true abnormalities, identify and exclude focal 18F-FDG uptake that occurs as a result of normal variants (e.g., excreted tracer in ureters, normal bowel wall activity), and guide subsequent colonoscopy and biopsy.
Virtual colonoscopy using thin-slice CT and volumetric display of the CT dataset is a new approach for the screening of patients at risk for colon cancer (37,38). It is conceivable that the combination of virtual colonoscopy with 18F-FDG PET and combined PET/CT will add specificity to this new method by selectively identifying only hypermetabolic polyps, which would be expected to carry a higher risk for malignant degeneration. This is the subject of ongoing studies.
Summary of PET/CT in Gastrointestinal Malignancies
PET/CT has already proven useful in several small patient series. Based on preliminary observations PET/CT fusion contributes critical information in 30%–40% of patients as compared with PET alone. Ongoing and future studies will refine its exact place in the diagnostic work-up of patients with gastrointestinal malignancies and address how often PET/CT can eliminate the need for other imaging studies that are currently performed for the staging or detection of recurrence in these patients.
Pancreatic Carcinoma
18F-FDG PET is useful in distinguishing between benign and malignant pancreatic (39) lesions and appears superior to CT for this purpose in the evaluation of pancreatic masses and cystic tumors (40–42). A rigid comparison with newer CT and MR techniques has not been performed. PET/CT fusion imaging provides additional benefit over PET imaging alone by classifying correctly as benign or malignant those lesions that were considered equivocal on PET alone (43). PET/CT can affect patient management, because it can guide biopsies to the metabolically most active part of a pancreatic tumor. However, because of inaccuracies related to small tumor burden, low metabolism, or mucinous histology, it is unlikely that 18F-FDG PET or PET/CT will be able to provide a definitive and satisfactory answer in most patients with suspected pancreatic malignancy. Rather, most patients with a clinically resectable pancreatic mass who are considered to have a low operative risk will proceed directly to surgery. CT is also performed to assess the resectability of pancreatic tumors. PET/CT as it is currently performed in most institutions cannot address this question. To make a difference, the CT portion of the study would have to be performed with proper use of intravenous contrast and thin-slice technique (dual phase, 120 kV, 200 mAs, 1-mm slice).
MISCELLANEOUS TUMORS
Musculoskeletal Primary Tumors and Metastases
PET/CT is helpful in improving the accuracy of 18F-FDG PET in the detection of osseous metastases and in differentiation of osseous lesions from lesions in adjacent soft tissue (44,45). The differentiation between osseous and extraosseous lesions is a potentially important issue in the staging of many malignancies (e.g., lung cancer and malignant lymphoma, in which the presence of osseous lesions indicates M1 or stage IV diseases that are associated with poor prognosis and, therefore, usually necessitate a change in patient management). If lesions are indeed located in the skeleton, the CT component of the study usually will demonstrate osteoblastic or osteolytic lesions or, by exactly localizing increased 18F-FDG uptake, confirm the presence of previously unnoted insufficiency fractures (Fig. 15), osteochondrosis, or facet joint arthritis. In rare instances of normal or nearly normal CT, fusion images can demonstrate disease at unsuspected sites or can guide further evaluation with MRI or biopsy.
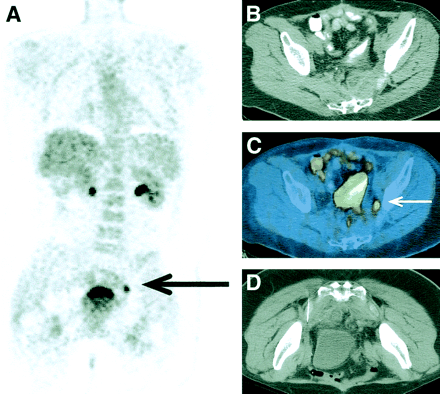
Patient with renal cell carcinoma. (A, B) Coronal and transaxial PET image showed abnormal 18F-FDG uptake in right sacral ala and right iliac bone, as well as adjacent soft tissue. In addition, there was smaller area of abnormal 18F-FDG uptake in left sacral ala. (C) Fusion images showed large lytic metastasis in left sacrum and ilium, expanding into adjacent musculature. However, abnormal 18F-FDG uptake on left was result of insufficiency fracture (clearly seen on fusion images) and not metastasis in this location.
PET/CT fusion is also helpful in interpreting otherwise unexplained 18F-FDG uptake in the skeleton or soft tissue in cancer patients (e.g., benign bone tumors, old fractures, myositis ossificans, rhabdomyolysis) that would otherwise require further investigations in a separate study.
18F-FDG PET is a valuable imaging modality in the staging and detection of recurrent disease in patients with musculoskeletal sarcomas. Occasionally, an 18F-FDG PET study will demonstrate sites of abnormal tracer uptake for which there is no clear correlation on CT, MRI, or bone scan or for which the anatomic imaging modalities cannot distinguish between posttreatment changes and potential recurrent disease in soft tissue. In these cases, PET/CT fusion images are extremely valuable in guiding further interventions (Fig. 16).
Patient (22-y-old woman) who was 2 y post resection of undifferentiated sarcoma from the left back. PET was performed for surveillance in this patient at high risk for recurrent/metastatic disease. (A) The coronal PET image showed focally increased 18F-FDG uptake in left back. (B) A bone scan showed no abnormality. (C) CT showed no abnormality. (D) The fusion image clearly localized lesion with abnormal 18F-FDG uptake in musculature of left upper back. Subsequent surgery, based on PET/CT findings, proved recurrent sarcoma.
Gynecologic Cancers
Selected case reports in patients with ovarian and fallopian tube carcinoma have demonstrated the advantage of PET/CT fusion imaging in these disease entities. Abnormalities frequently demonstrate increased 18F-FDG uptake but cannot be detected by CT. PET/CT fusion imaging is therefore essential for localizing the tumor and directing further management (46,47).
Peritoneal disease can be detected by 18F-FDG PET. In a small group of patients with suspected recurrent ovarian cancer, PET/CT appeared to be more sensitive than CT alone in the detection of abdominal/peritoneal metastases (48); additional lesions were identified in 16 of 20 patients. In another study in a similar patient population, PET/CT had a specificity of 100% and a sensitivity of 75% for detecting peritoneal implants (49). It is obvious that a contrast-enhanced spiral CT in thin section technique, and possibly the multiplanar review of CT images, will detect smaller peritoneal implants better (49,50) than low-dose noncontrast CT as it is routinely performed as part of PET/CT studies. On the other hand, the detection of peritoneal metastases by PET depends on intensity of tracer uptake and, to some degree, on lesion size. It therefore appears that in this patient group the PET/CT would be performed best with contrast-enhanced CT of full diagnostic quality (e.g., 120 kV, 140–200 mAs, thin-slice technique).
Leiomyosarcoma of the uterus is a rare malignancy. The tumor exhibits intense 18F-FDG uptake, so that PET can be used in the follow-up of these patients to detect recurrent disease. An example is shown in Figure 17. PET/CT identified and localized foci of increased 18F-FDG uptake adjacent to the urinary bladder, consistent with local recurrence. The patient underwent tumor resection and intraoperative brachytherapy on the next day, and histopathology revealed recurrent leiomyosarcoma.
Patient with recurrent leiomyosarcoma. Imaging was performed for surveillance in this patient at high risk for tumor recurrence. (A) Coronal PET image showed abnormal 18F-FDG uptake in right lower pelvis. (B) Transaxial CT showed nonspecific soft tissue posterior to urinary bladder. (C) Fusion images showed abnormal 18F-FDG uptake in this lesion. Patient subsequently underwent resection of recurrent leiomyosarcoma, combined with intraoperative brachytherapy.
CONCLUSION
Most published studies are retrospective in nature and have certain limitations. Nevertheless, even in the phase of initial evaluation, PET/CT has already proven useful in the assessment of patients with lymphoma, melanoma, gastrointestinal and gynecologic malignancies, and sarcomas. Future studies will have to address the advantages of PET/CT for specific clinical questions, compare results with those with PET alone or visual correlation of PET with CT/MRI, evaluate the cost-effectiveness of PET/CT, and assess its effects on patient management. Undoubtedly, PET/CT will be of particular advantage in the application of newer PET radiotracers that are more specific than 18F-FDG. The more specific the tracer, the more selective will be the tissue uptake or receptor binding. Anatomic information provided by fusion images will be essential to assure the clinical application of such new imaging agents. In summary, although many questions remain open, the future for PET/CT in clinical oncology and oncologic research will certainly be bright.
Footnotes
Received Nov. 12, 2003; revision accepted Nov. 21, 2003.
For correspondence or reprints contact: Heiko Schöder, MD, Department of Radiology/Nuclear Medicine, Memorial Sloan-Kettering Cancer Center, 1275 York Ave., New York, NY 10021.
E-mail: schoders{at}mskcc.org