Abstract
Cardiac PET using 18F-FDG under fasting conditions (fasting 18F-FDG PET) is a promising technique for identification of cardiac sarcoidosis and assessment of disease activity. The aim of this study was to investigate the usefulness of fasting 18F-FDG PET in detecting inflammatory lesions of cardiac sarcoidosis from a pathophysiologic standpoint. Methods: Twenty-two patients with systemic sarcoidosis were classified into 2 groups of 11 each according to the presence or absence of sarcoid heart disease. Cardiac sarcoidosis was diagnosed according to the Japanese Ministry of Health and Welfare guidelines for diagnosing cardiac sarcoidosis with the exception of scintigraphic criteria. Nuclear cardiac imaging with fasting 18F-FDG PET, 99mTc-methoxyisobutylisonitrile (99mTc-MIBI) SPECT, and 67Ga scintigraphy were performed in all patients. PET and SPECT images were divided into 13 myocardial segments and the standardized uptake value (SUV) of 18F-FDG was calculated and defect scores (DS) for 99mTc-MIBI uptake were assessed for each segment. The total SUV (T-SUV) and total DS (TDS) were calculated as the sum of measurements for all 13 segments, and the diagnostic accuracy of fasting 18F-FDG PET was compared with that of the other nuclear imaging modalities. In addition, pathophysiologic relationships between inflammatory activity and myocardial damage were examined by segmental comparative study using the SUV and DS. Results: In patients with cardiac sarcoidosis, fasting 18F-FDG PET revealed a higher frequency of abnormal myocardial segments than 99mTc-MIBI SPECT (mean number of abnormal segments per patient: 6.6 ± 3.0 vs. 3.0 ± 3.2 [mean ± SD], P < 0.05). The sensitivity of fasting 18F-FDG PET in detecting cardiac sarcoidosis was 100%, significantly higher than that of 99mTc-MIBI SPECT (63.6%) or 67Ga scintigraphy (36.3%). The accuracy of fasting 18F-FDG PET was significantly higher than 67Ga scintigraphy. The T-SUV demonstrated a good linear correlation with serum angiotensin-converting enzyme levels (r = 0.83, P < 0.01), and the TDS showed a significant negative correlation with the left ventricular ejection fraction (r = −0.82, P < 0.01). In abnormal myocardial segments on the nuclear scan, the SUV showed a significant negative correlation with the DS (r = −0.63, P < 0.0001). Conclusion: This study suggests that fasting 18F-FDG PET can detect the early stage of cardiac sarcoidosis, in which fewer perfusion abnormalities and high inflammatory activity are noted, before advanced myocardial impairment.
Sarcoidosis is a systemic disorder of unknown cause that is characterized by its pathologic hallmark, the noncaseating granuloma. Although the disease is generally associated with a low mortality rate, cardiac involvement may carry a poor prognosis. Myocardial involvement is present in 20%–76% of patients with sarcoidosis (1,2) and is responsible for as many as 77% of deaths from the disease (3). Although endomyocardial biopsy is required for definitive diagnosis of cardiac sarcoidosis, it is invasive and may lack sensitivity because myocardial involvement is not homogeneous. Scintigraphy using 67Ga, 201Tl, and 99mTc-methoxyisobutylisonitrile (99mTc-MIBI) are also used to detect cardiac involvement in patients with sarcoidosis (4–6). An abnormal perfusion area on 201Tl or 99mTc-MIBI SPECT is considered as impaired myocardium, although myocardial perfusion defects are not specific to sarcoidosis and can occur with ischemic heart disease or other cardiomyopathies (4,5). Furthermore, although accumulation of 67Ga is considered an indicator of inflammatory change, 67Ga scintigraphy is an insensitive method for detection of cardiac involvement in sarcoidosis (7). Recent studies have revealed that 18F-FDG PET under fasting conditions (fasting 18F-FDG PET) is a useful method for identification of cardiac sarcoidosis and for assessment of disease activity (7,8). However, to our knowledge, no investigations have been published with regard to the usefulness of fasting 18F-FDG PET in detecting cardiac sarcoidosis that take into account pathophysiologic considerations. The present study was designed to evaluate the usefulness of fasting 18F-FDG PET in detecting inflammatory lesions of cardiac sarcoidosis from a pathophysiologic standpoint, based on correlative analysis between the uptake pattern in combined nuclear imaging studies and the clinical and histologic findings in patients with cardiac sarcoidosis.
MATERIALS AND METHODS
Patients and Healthy Subjects
Patients.
Twenty-two patients (3 men, 19 women; mean age, 62.1 y; range, 37–78 y) with active sarcoidosis were prospectively recruited into this study. Diagnosis was confirmed histologically by the presence of noncaseating granulomas on intraabdominal lymph node biopsy (patient 4), transbronchial lung biopsy (patients 5, 6, and 12–16), scalene node biopsy (patients 7, 8, 17, and 18), skin biopsy (patients 9–11 and 19–22), or endomyocardial biopsy (patients 1–3). No patients showed evidence of diabetes mellitus. Patient details are given in Table 1. The 22 patients were classified into 2 groups according to the presence or absence of clinically well-documented sarcoid heart disease. Eleven patients had evidence of cardiac sarcoidosis (group A) and the other 11 patients did not (group B). Cardiac sarcoidosis was diagnosed according to the relevant Japanese Ministry of Health and Welfare guidelines for diagnosing cardiac sarcoidosis described in Table 2 (9), but criterion 2c was not used to avoid the bias from the analysis. The serum angiotensin-converting enzyme (ACE) level was measured in all patients treated without ACE inhibitors. Endomyocardial biopsy was performed in all patients in group A, and 2 or 3 specimens were obtained from the left ventricle. Diagnosis of sarcoid heart disease, based on the presence of noncaseating granulomas on endomyocardial biopsy (histologic cardiac sarcoidosis), was made in 3 patients (patients 1–3) who showed atrioventricular block or intractable ventricular tachycardia. In the patients without histologic evidence on endomyocardial biopsy, sarcoid heart disease was diagnosed based on the presence of severe unexplained cardiac problems, including echocardiographically proven ventricular dysfunction, serious ventricular arrhythmia (≥grade 2, Lown’s classification), or second- or third-degree atrioventricular block (patients 4–11: clinical cardiac sarcoidosis). Cardiac catheterization, including selective coronary angiography, was performed in patients with sarcoid heart disease and failed to reveal atherosclerotic stenoses.
Characteristics of 22 Patients
Guidelines for Diagnosis of Cardiac Sarcoidosis from Japanese Ministry of Health and Welfare
Healthy Subjects.
Seven healthy volunteers (mean age, 46.0 y; range, 37–65 y) were studied to obtain reference values for the standardized uptake value (SUV) of 18F-FDG in the heart.
Written informed consent was obtained from all patients and volunteers, and the study protocol was approved by the institutional ethics committee and radiation protection authorities.
Imaging Protocol
Within a 2-wk period after entering the study, all patients underwent 18F-FDG myocardial PET, 99mTc-MIBI myocardial SPECT, and 67Ga scintigraphy.
18F-FDG PET.
Whole-body 18F-FDG dedicated PET was performed with a SET 2400W (Shimadzu Corp.) with a 59.5-cm transaxial field of view and 20-cm axial view that produced 63 image planes spaced 3.125 mm apart. All patients fasted for at least 12 h before the examination, and plasma glucose levels were measured before injection of the tracer. Simultaneous emission-transmission scans were obtained 1 h after injection of approximately 200 MBq (5.4 mCi) of 18F-FDG. The performance characteristics of this scanner and processing methods have been described previously (10).
99mTc-MIBI SPECT and 67Ga Scintigraphy.
In all patients, 99mTc-MIBI SPECT for myocardial perfusion imaging and 67Ga scintigraphy were performed. Myocardial SPECT data acquisition commenced 40–50 min after intravenous injection of the tracer (600 MBq of 99mTc-MIBI) during rest and after at least 12 h of fasting. To remove 99mTc-MIBI accumulated in the gallbladder, all patients drank milk between tracer injection and image acquisition.
With a triple-head SPECT γ-camera (Prism 3000; Marconi/Shimadzu) equipped with low-energy, general-purpose collimators, a total of 20 projection images were obtained over 360° in 6° increments, at 50 s per view. Myocardial SPECT data were processed by filtered backprojection (Butterworth filter, cutoff frequency at 0.2 cycle/pixel; order, 4).
67Ga scintigraphic imaging was performed 72 h after intravenous injection of 74 MBq of 67Ga citrate. Views were obtained with a large-field γ-scintillation camera (Prism 2000; Picker) with 3 photopeaks (93, 184, and 296 keV) equipped with a medium-energy, general-purpose collimator. Anterior and posterior views of the thorax were obtained.
Image Analysis
Functional images of the SUV were produced from attenuation-corrected images, with reference to the injected dose of 18F-FDG, body weight, and cross-calibration factors between PET and the dose calibrator. SUV was defined as follows:
 For the analysis of myocardial accumulation of 18F-FDG, a polygonal region of interest (ROI) for each of the 13 myocardial segments (Fig. 1) was placed manually on SUV images of short-axis and vertical long-axis slices. The total SUV (T-SUV) was calculated as the sum of the values for all 13 segments in all 22 patients.
For the analysis of myocardial accumulation of 18F-FDG, a polygonal region of interest (ROI) for each of the 13 myocardial segments (Fig. 1) was placed manually on SUV images of short-axis and vertical long-axis slices. The total SUV (T-SUV) was calculated as the sum of the values for all 13 segments in all 22 patients.
Short-axis and vertical long-axis slices of myocardial PET and SPECT images were divided into 13 segments.
Semiquantitative visual analysis of the SPECT data was performed. Short-axis and vertical long-axis slices of the SPECT images were divided into 13 segments in the same manner as for PET. Segmental uptake of the tracer was graded by visual assessment using a 4-point scoring system (0 = normal; 1 = mild reduction; 2 = moderate reduction; and 3 = severe reduction in tracer uptake or absence of tracer uptake). The total defect score (TDS) was calculated as the sum of the scores for all 13 segments.
Abnormal myocardial 67Ga uptake was considered present when abnormal intensity was clearly observed in anterior projections of the heart.
SPECT and scintigraphic images were interpreted independently by 2 experienced nuclear physicians who had no prior information about the clinical and histopathologic data. The examination was followed by discussion until a consensus was reached.
Statistical Analysis
Data are expressed as mean ± SD. Sensitivities of fasting 18F-FDG PET, 99mTc-MIBI SPECT, and 67Ga scanning for detecting myocardial lesions of sarcoidosis were compared using the Fisher exact test. The Student t test for unpaired data was used to examine differences between groups. Correlations between the 2 values were analyzed by simple linear regression analysis or the Spearman rank correlation test. Differences were considered significant when P < 0.05.
RESULTS
Blood Glucose
Fasting plasma blood glucose concentrations were 72–104 mg/dL in group A, 74–104 mg/dL in group B, and 75–103 mg/dL in healthy volunteers. No significant differences were apparent between groups with respect to the mean blood glucose level.
Normal Segmental SUV
In healthy volunteers, the mean + 2 SD of the SUV was calculated for each of the 13 myocardial segments to determine the normal upper level of the SUV (Table 3). A segmental SUV greater than this level was defined as abnormal in this study. No statistically significant differences were observed between the SUV for each segment among healthy volunteers.
SUV of 18F-FDG for Each of 13 Myocardial Segments in Healthy Volunteers
Frequency of Abnormal Segments
A total of 143 myocardial segments were analyzed both in group A and in group B. The results of segmental analysis of myocardial nuclear imaging using 18F-FDG PET and 99mTc-MIBI SPECT are summarized in Table 4.
Results of Myocardial Nuclear Imaging
In group A, abnormal uptake of 18F-FDG was observed in all patients. Among the total 143 myocardial segments examined in group A patients, 73 segments exhibited an abnormal high SUV (range, 2.51–14.70; mean, 6.16). A segmental perfusion abnormality on 99mTc-MIBI SPECT occurred in 33 segments (mean DS = 2.1; range, 1–3) in 7 patients. The mean number of abnormal segments per patient (frequency of abnormal segments) was 6.6 ± 3.0 (range, 3–13) on 18F-FDG PET, which was significantly higher than that on 99mTc-MIBI SPECT (3.0 ± 3.2; range, 0–10) (P < 0.05).
Histologic and Clinical Cardiac Sarcoidosis
The frequency of segments demonstrating abnormal 18F-FDG uptake in patients with histologic cardiac sarcoidosis (patients 1–3) was 4.7 segments per patient (range, 4–6), which was lower than that in patients with clinical cardiac sarcoidosis (7.4; range, 3–13); however, this trend did not reach statistical significance (Fig. 2A). The frequency of segments with abnormal 99mTc-MIBI uptake in patients with histologic cardiac sarcoidosis was significantly higher than that in those with clinical cardiac sarcoidosis (Fig. 2B). The mean T-SUV was significantly lower in patients with histologic cardiac sarcoidosis as compared with that in those with clinical cardiac sarcoidosis (Fig. 2C). Conversely, the mean TDS was significantly higher in patients with histologic cardiac sarcoidosis as compared with that in those with clinical cardiac sarcoidosis (Fig. 2D).
Comparison of nuclear measurements between histologically diagnosed cardiac sarcoidosis (HCS) and clinically diagnosed cardiac sarcoidosis (CCS). Frequency of abnormal segments on 99mTc-MIBI SPECT and TDS in HCS (white bar) was significantly higher than that in CCS (black bar) (B and D). In contrast, frequency of abnormal segments on fasting 18F-FDG PET and T-SUV in HCS were lower than those in CCS, although significant difference could be observed only for T-SUV (A and C). NS = not significant.
Relationships Between Imaging Abnormality and Clinical Data
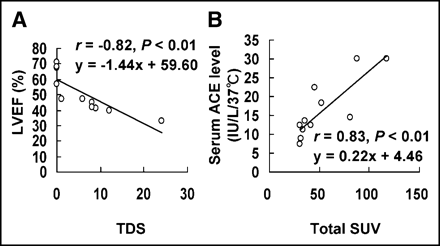
Table 4 summarizes the TDS on 99mTc-MIBI SPECT and the T-SUV on 18F-FDG PET for each patient. Among group A patients, a statistically significant correlation was observed between the TDS and left ventricular ejection fraction (LVEF) (r = −0.82, P < 0.01) (Fig. 3A), whereas no significant correlation was found between the T-SUV and LVEF. Furthermore, the T-SUV and serum ACE level showed a good linear correlation (y = 0.22x + 4.46; r = 0.83; P < 0.01) (Fig. 3B), but the TDS was not correlated with serum ACE. No significant correlation was found between the TDS and T-SUV (data not shown).
Relationship between nuclear measurements (T-SUV and TDS) and clinical data (LVEF and serum ACE level) in patients with cardiac sarcoidosis (group A). Statistically significant correlations were observed between TDS and LVEF (A) and between T-SUV and serum ACE level (B).
Segmental Analysis
We also performed particular studies on the myocardial segments with abnormal nuclear imaging findings in order to clarify the clinical pathophysiologic relationships between inflammatory activity and myocardial damage in myocardial lesions of cardiac sarcoidosis. We classified myocardial segments into the following 4 types based on the findings of 2 nuclear imaging techniques: normal segment = normal 18F-FDG uptake with normal 99mTc-MIBI perfusion (n = 54), matched abnormal segment = abnormal 18F-FDG uptake with abnormal 99mTc-MIBI perfusion (n = 17), mismatched segment = abnormal 18F-FDG uptake with normal 99mTc-MIBI perfusion (n = 56), and inverse mismatched segment = normal 18F-FDG uptake with abnormal 99mTc-MIBI perfusion (n = 16). Further studies were performed on the 3 types of abnormal segments (matched abnormal, mismatched, and inverse mismatched segment) as described below.
SUV in Segments with Abnormal 18F-FDG Uptake.
SUV was significantly lower in matched abnormal segments (range, 2.51–8.30) than in mismatched segments (range, 3.18–14.70) (mean values, 4.77 ± 2.06 vs. 6.58 ± 3.00, P < 0.01). SUV was significantly correlated with DS in the segments with abnormal 18F-FDG uptake (r = −0.33, P < 0.01) (Fig. 4A).
Correlation between SUV and DS in segments with abnormal 18F-FDG uptake (A), those with abnormal 99mTc-MIBI perfusion (B), and those with abnormal 18F-FDG uptake or 99mTc-MIBI defect (C). Significant negative correlations were observed in all types of abnormal segment.
DS in Segments with Abnormal 99mTc-MIBI Perfusion.
The mean DS of matched abnormal segments was significantly lower than that of inverse mismatched segments (1.82 ± 0.53 vs. 2.31 ± 0.48, P < 0.01). Significant correlations were found between the DS and SUV in segments with abnormal 99mTc-MIBI perfusion (r = −0.48, P < 0.01) (Fig. 4B).
Relationships Between SUV and DS in Abnormal Segments.
In the 3 types of abnormal segments (matched abnormal, mismatched, and inverse mismatched), a statistically significant negative correlation was found between SUV and DS (r = −0.63, P < 0.0001) (Fig. 4C).
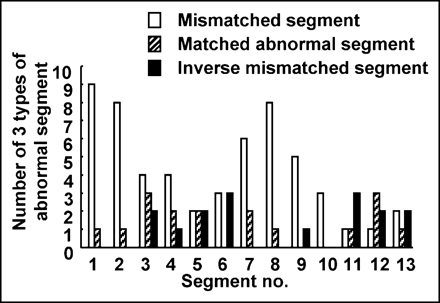
Localization and Distribution of Abnormal Segments.
Localization of the 3 types of abnormal segment is summarized in Figure 5. Mismatched segments were frequently observed in the basal and mid anteroseptal-lateral wall of the left ventricle (LV), whereas inverse mismatched segments had a tendency to localize in the septal and inferior wall of the LV and matched abnormal segments showed no tendency to localize. Abnormal segments showed discontinuous distribution in only 2 patients (patients 7 and 11), being distributed continuously in the other 9 patients.
Localization of 3 types of abnormal segment.
Sensitivity and Specificity
Using the guidelines for diagnosing cardiac sarcoidosis provided by the Japanese Ministry of Health and Welfare as a gold standard, we calculated the sensitivity, specificity, and accuracy of 18F-FDG PET, 99mTc-MIBI SPECT, and 67Ga scintigraphy for the detection of cardiac sarcoidosis (Table 5). Abnormal 18F-FDG uptake was observed in all group A patients, resulting in a sensitivity of 100%, which was significantly higher than that of 99mTc-MIBI SPECT or 67Ga scintigraphy (63.6% and 36.4%, respectively). With regard to specificity, no significant differences were found between imaging modalities. The accuracy of 18F-FDG PET, 99mTc-MIBI SPECT, and 67Ga scintigraphy was 95.5% (21/22), 81.8% (18/22), and 68.2% (15/22), respectively. Although the accuracy of 18F-FDG PET was higher than that of the other nuclear imaging modalities, a significant difference was observed only with respect to 67Ga scintigraphy.
Diagnostic Performance of Fasting 18F-FDG PET, 99mTc-MIBI SPECT, and 67Ga Scintigraphy
DISCUSSION
The main results of this study can be summarized as follows: (a) Fasting 18F-FDG PET can be performed in patients with sarcoidosis for the detection of myocardial abnormalities and results in more frequent detection of abnormalities than either 99mTc-MIBI SPECT or 67Ga scintigraphy. (b) Fasting 18F-FDG PET can provide useful information with regard to the extent and activity of inflammation in cardiac sarcoidosis in the particular condition. (c) Semiquantitative analysis using fasting 18F-FDG PET in combination with 99mTc-MIBI SPECT can simultaneously evaluate regional inflammatory activity and perfusion abnormalities. In this regard, our results demonstrated fewer perfusion abnormalities in those myocardial segments exhibiting high inflammatory activity.
Several studies have previously shown the diagnostic value of 18F-FDG PET in myocardial involvement of sarcoidosis (7,8). However, to our knowledge, this is the first study concerning the diagnostic accuracy of fasting 18F-FDG PET in detecting inflammatory lesions of cardiac sarcoidosis from a pathophysiologic standpoint, based on correlative analysis between uptake patterns shown by combined nuclear imaging studies and clinical and histologic findings.
Diagnostic Accuracy
Evidence of increased glucose metabolism in inflamed tissue has been demonstrated by various experimental studies (11,12). Newsholme and Newsholme demonstrated that inflammatory cells in an activated state produce 7- to 8-fold higher levels of ATP when compared with those under baseline conditions (13). Mauel also showed significantly increased glucose oxidation through the hexose monophosphate shunt pathway when murine macrophages were exposed to dilutions of the bacterial extract OM-86BV (14). Macrophages are known to have high rates of protein secretion and membrane recycling. Indeed, rapidly dividing cells, such as activated inflammatory cells, have high glycolytic activity to satisfy their large energy demands, a fact that is supported by evidence of increased metabolic activity noted during immunologic responses (13). Histologic studies of biopsy specimens suggest that macrophages, which contribute to granuloma formation, are activated (15). This may explain in part why granulomatous lesions demonstrate high uptake of 18F-FDG.
FDG is a glucose analog that traces the transmembranous transport and hexokinase-mediated phosphorylation of glucose. FDG-6-phosphate is then effectively trapped within the myocyte, because the sarcolemma is relatively impermeable to this intermediate, which is a poor substrate for further metabolism by either glycolytic or glycogen-synthetic pathways, and because dephosphorylation of FDG-6-phosphate is thought to be quite slow. The regional distribution of myocardial activity, assessed 40–60 min after intravenous administration of FDG, is believed to be related to overall (anaerobic and aerobic) regional glycolytic flux (16).
In normal myocardium, metabolism is primarily oxidative and utilizes various admixtures of substrates (free fatty acids, glucose, and lactate). The proportional contribution of each substrate to overall oxidative metabolism is dependent on multiple factors, including arterial substrate content, the hormonal milieu, and the temporal relationship to a myocardial ischemic insult (17,18). Under fasting conditions, plasma insulin levels fall, resulting in reduced transport of glucose into myocytes and an increase in the availability of free fatty acids secondary to increased lipolysis in peripheral adipose tissue. Under these conditions, free fatty acids become the preferred energy substrate for oxidative metabolism, whereas the contribution of glucose to total energy production via oxidative metabolism is decreased. Elevation of plasma fatty acids leads to inhibition of absolute glucose utilization by the heart (19) and results in subtraction of background myocardial 18F-FDG uptake, causing more distinct visualization of 18F-FDG accumulation in inflammatory lesions. Therefore, small absolute differences in glucose utilization between inflamed and normal myocardium may appear relatively large under these conditions. It is reasonable to consider fasting 18F-FDG PET as a suitable method to detect the metabolic sequelae of inflammatory lesions of cardiac sarcoidosis because it accentuates the difference between inflamed tissue (increased accumulation of 18F-FDG) and normal tissue (less uptake). For these reasons, fasting 18F-FDG PET was shown to be preferable in this study, demonstrating higher performance in detecting myocardial involvement of sarcoidosis as compared with the other nuclear diagnostic imaging modalities. In addition to high spatial resolution, the particular metabolic setting described here was considered to play an important role in the superior diagnostic performance of fasting 18F-FDG PET in detecting cardiac sarcoidosis.
Pathophysiologic Considerations
It is generally accepted that interstitial pneumonitis with mononuclear cell infiltration of macrophages and lymphocytes represents a very early lesion, possibly the initial lesion, in pulmonary sarcoidosis, predating the appearance of characteristic epithelioid granulomas. Several clinical and experimental investigations support this view of histologic development in sarcoidosis, confirming that epithelioid granuloma formation is preceded by a diffuse mononuclear cell infiltration in all sites where granulomas are found (20–23). It was recently reported that monocyte chemoattractant protein-1, an important mediator of monocyte infiltration in the monocyte/macrophage-mediated inflammatory process (24–26), was markedly increased in the early stage of pulmonary sarcoidosis and was significantly correlated with serum ACE levels (27). In contrast, as the granuloma matures, the numbers of epithelioid cells increase and the numbers of monocytes, macrophages, and lymphocytes decline (20,28). In patients with progressive lesions containing numerous granulomas, alveolitis is minimal or absent (20). Additionally, a well-developed characteristic epithelioid granuloma is encircled by antiinflammatory T-lymphocytes, which fulfill the function of suppressing granulomatous formation (29). This evidence strongly indicates that the inflammatory reaction is more prominent in the early stage of sarcoidosis compared with the advanced stage of the disease in which characteristic granulomas develop. This results in a higher population of activated macrophages in the early disease. It has been clearly demonstrated that 18F-FDG accumulates in macrophages and reactive lymphocytes of inflammatory tissue (30). Activated monocytes/macrophages and T-lymphocytes in inflammatory tissue use glucose as an energy source for chemotaxis and phagocytosis (31) and can increase both oxygen consumption and glucose metabolism in response to immune reactions (32). However, clinical investigations using PET that take into account the pathophysiology of cardiac sarcoidosis have not yet been reported.
In the segmental analysis of this study, normal segments represented myocardium with normal perfusion and no inflammatory lesion, mismatched segments represented normal perfusion and active inflammatory lesion (early stage of disease), matched abnormal segments represented abnormal perfusion and active inflammatory lesion (advanced stage of disease), and inverse mismatched segments represented abnormal perfusion and no inflammatory lesion (end-stage of disease). Myocardial segments with a DS of 3 on 99mTc-MIBI imaging are considered to represent fibrotic degeneration and 6 segments showed a DS of 3 in the present study. Interestingly, 5 of these 6 segments showed a normal SUV of 18F-FDG and the SUV was almost normal in the remaining segment, indicating that, in end-stage myocardium, active inflammatory lesions are no longer present. This premise is supported by the striking finding that the SUV of affected myocardial segments showed a negative correlation with DS.
On the other hand, comparative study between histlogic cardiac sarcoidosis and clinical cardiac sarcoidosis demonstrated that the mean T-SUV of a patient with histologic cardiac sarcoidosis was significantly lower than that of clinical cardiac sarcoidosis. In contrast, the mean TDS of histologic cardiac sarcoidosis was significantly higher than that of clinical cardiac sarcoidosis. This evidence might indicate that clinical cardiac sarcoidosis predominantly represents an early stage of the disease with mononuclear cell infiltration containing abundant macrophages preceding formation of epithelioid granuloma and that histologic cardiac sarcoidosis mainly represents a mature stage of the disease with numerous granulomas. This stage would result in a high prevalence of positive histologic findings on endomyocardial biopsy examination. However, positive findings on endomyocardial biopsy were also found in patients with localized lesions (patients 1 and 2). Because the number of patients with positive findings on endomyocardial biopsy in this study might be too small to draw conclusions from statistical analysis, studies should be performed in a larger population to confirm our results.
It is now apparent that sarcoid lesions can originate at different times (33,34) in the same organ. Therefore, the myocardium of patients with cardiac sarcoidosis would be expected to contain various stages of inflammatory lesions with varying severity. This speculation is supported by our finding that almost group A patients demonstrated various types of abnormal segments at the same time (Table 4). Our results (Table 4) also demonstrate that different stages in the natural history of cardiac sarcoidosis could be recognized in each patient according to the characteristic distribution of the 3 types of abnormal segment and the combined pattern of T-SUV and TDS. The patients in group A can be longitudinally classified from a pathophysiologic standpoint as follows: those in the early stage of disease (patients 5, 8, 9, and 11; high T-SUV with a TDS of 0), the progressive inflammatory stage (patient 4; very high T-SUV with low TDS), the peak active stage (patient 10; extremely high T-SUV with moderately high TDS), the progressive myocardial impairment stage (patient 6; very high T-SUV with high TDS), and the fibrosis-dominant, low-activity stage (patients 1–3 and 7; low T-SUV with high TDS). Patients 1 and 2 might represent those with localized lesions and patient 22 might have the early stage of the disease. Two representative cases (patient 9 [early stage] and patient 1 [fibrosis-dominant low-activity stage]) are shown in Figures 6 and 7.
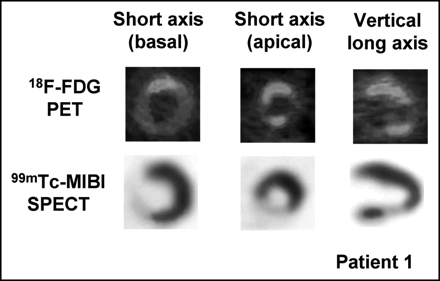
Fasting 18F-FDG PET images (top) and 99mTc-MIBI SPECT images (bottom) in patient 9. 18F-FDG PET revealed markedly increased uptake of 18F-FDG in segments 1–8, whereas 99mTc-MIBI SPECT revealed no abnormal findings.
Fasting 18F-FDG PET images (top) and 99mTc-MIBI SPECT images (bottom) in patient 1. 18F-FDG PET revealed slightly increased uptake of 18F-FDG in segments 1, 2, 4, and 12. 99mTc-MIBI SPECT clearly revealed moderate reduction of 99mTc-MIBI uptake in segments 5, 6, and 12 and severe reduction of 99mTc-MIBI uptake in segment 3.
In summary, these results indicate that myocardial segments with high inflammatory activity (high SUV level) show less myocardial involvement (lower DS), indicating the early stage of sarcoid lesions. Conversely, our results indicate that myocardial segments with severe myocardial involvement (high DS) considered to be progressive myocardial lesions show less inflammatory activity (low SUV level) and that end-stage myocardial fibrosis (DS of 3) no longer exhibits active inflammatory lesions (normal or near-normal SUV).
Significance of Fasting 18F-FDG PET in Cardiac Sarcoidosis
Conventional SPECT with 201Tl or 99mTc-MIBI is used to detect myocardial involvement in cardiac sarcoidosis. However, the abnormalities demonstrated in these SPECT images do not represent early lesions but, instead, signify impaired myocardium in the progressive stage. In contrast, abnormal 18F-FDG uptake on fasting 18F-FDG PET is seen in active inflammatory lesions at the early stage of cardiac sarcoidosis. Therefore, fasting 18F-FDG PET is a particularly appropriate imaging modality for detecting the early stage of myocardial lesions, in which high inflammatory activity precedes severe myocardial involvement, rather than the advanced stage of the disease. Clinically, this method might therefore represent a promising technique for diagnosis of the early stage of cardiac sarcoidosis and thus be valuable in providing adequate treatment. This could help prevent myocardial involvement and possible irreversible end-stage fibrosis.
On the other hand, the significance of rest scintigraphic perfusion defects remains in question. Granuloma with scar fibrosis or microcirculatory dysfunction has been considered as a cause for perfusion defects on myocardial SPECT in cardiac sarcoidosis (6,35,36). A previous study found that patients with idiopathic dilated cardiomyopathy exhibited heterogeneous myocardial glucose uptake (37), and a more recent study reported that increased uptake of 18F-FDG was found in myocardial segments with anaerobic metabolism in the patients with idiopathic dilated cardiomyopathy (38). This indicates that increased uptake of 18F-FDG positively correlates with ischemic involvement in the patients with idiopathic dilated cardiomyopathy. In cardiac sarcoidois, if vasoconstrictive ischemia of the microvasculature is induced by the inflammatory process and accounts for myocardial 99mTc-MIBI perfusion defects, myocardial perfusion abnormality would show a positive correlation with 18F-FDG uptake. However, in the present study, myocardial perfusion abnormality showed an impressive negative correlation with myocardial 18F-FDG uptake in patients with cardiac sarcoidosis who showed normal coronary angiographic findings. Although 99mTc-MIBI defects might be partially dependent on microvascular vasoconstriction, our data indicated that scar fibrosis was the main mechanism of the 99mTc-MIBI perfusion defect.
Limitations
The use of high-dose corticosteroids has been encouraged in the hope that it may alter the course of the cardiac sarcoidosis if started early (39,40). In this study, the response of fasting 18F-FDG PET to steroid treatment in patients with cardiac sarcoidosis was not investigated. A recent study found that cardiac 13N-NH3/18F-FDG PET under fasting conditions was useful both in identification of cardiac involvement of sarcoidosis and in following inflammatory response to therapy (7). However, to our knowledge, there are no pathophysiologic studies with segmental analysis that investigate the usefulness of fasting 18F-FDG PET for the evaluation of steroid therapy in cardiac sarcoidosis. Serial and longitudinal pathophysiologic investigation of fasting 18F-FDG PET using segmental analysis of the natural and treated course of sarcoidosis is necessary to further delineate the clinical usefulness of this method in the management of this disease.
CONCLUSION
In this study, we investigated the usefulness of fasting 18F-FDG PET for the detection of myocardial involvement in patients with cardiac sarcoidosis detected using orthodox diagnostic criteria for cardiac sarcoidosis. Fasting 18F-FDG PET detected abnormalities more frequently than either 99mTc-MIBI SPECT or 67Ga scintigraphy in patients with cardiac sarcoidosis. Segmental nuclear analysis using combined imaging with fasting 18F-FDG PET and 99mTc-MIBI SPECT suggested that fasting 18F-FDG PET could detect the early stage of cardiac sarcoidosis that is associated with less perfusion abnormalities and greater active inflammation. These results indicate that fasting 18F-FDG PET is an appropriate imaging modality suitable for the diagnosis of cardiac sarcoidosis in the clinical setting, particularly in the early stage of myocardial involvement before advanced myocardial impairment.
Acknowledgments
We thank Kunio Matsubara (Division of Cyclotron Laboratory in Gunma University School of Medicine) for his technical assistance.
Footnotes
Received Apr. 28, 2004; revision accepted Jul. 21, 2004.
For correspondence or reprints contact: Wataru Okumura, MD, Second Department of Internal Medicine, Gunma University School of Medicine, 3-39-22 Showamachi Maebashi, Gunma, 371-8511 Japan.
E-mail: wokumura-circ{at}umin.ac.jp