Abstract
This study is a retrospective analysis of 124 differentiated thyroid cancer patients who underwent dosimetric evaluation using MIRD methodology over a period of 15 y. The objectives of the study were to demonstrate the clinical use of dosimetry-guided radioactive iodine ([RAI] 131I) treatment and the safe and effective application of a 3-Gy bone marrow (BM) dose in patients with differentiated thyroid cancer. Methods: Tumor and BM dose estimates were obtained. The administered activity that would deliver a maximum safe dose to the organ at risk (red BM or lungs) was determined as well as the resulting doses to the metastases. The clinical benefit of an individual RAI treatment was predicted on the basis of the dose estimates and the expected therapeutic response. Each patient’s response to treatment was assessed clinically and by monitoring the hematologic profile. Results: One hundred twenty-four patients underwent 187 dosimetric evaluations. One hundred four RAI treatments were performed. A complete response at metastatic deposits was attained with absorbed doses of >100 Gy. No permanent BM suppression was observed in patients who received absorbed doses of <3 Gy to BM. The maximum administered dose was 38.5 GBq (1,040 mCi) with the BM dose limitation. Conclusion: Dosimetry-guided RAI treatment allows administration of the maximum possible RAI dose to achieve the maximum therapeutic benefit. Estimation of tumor dose rates helps to determine the curative versus the palliative intent of the therapy.
The optimal radioactive iodine (RAI) dose (administered activity) in the treatment of differentiated thyroid cancer (DTC) has been a subject of controversy since its first use by Seidlin et al. (1) in 1946. A dosimetric approach and administration of the maximum safe dose were first introduced in 1962 by Benua et al. (2), who observed that repeated subtherapeutic doses of RAI might induce dedifferentiation and loss of iodine-concentrating ability of tumors. The dose-limiting toxicity of RAI treatment is mainly on the bone marrow (BM), and the limit has been set as the dose (administered activity) that delivers 2 Gy (200 rad) to the blood as an equivalent of BM and whole-body retention of <4.44 GBq (<120 mCi) at 48 h (3).
There has been a significant improvement in dosimetric techniques over the past decades. Earlier dosimetric techniques involved blood and urine measurements. Image- based whole-body dose determinations have remarkably improved the accuracy and reproducibility of dosimetric calculations. The development of MIRD methodology has yielded a new paradigm in dosimetry (4). MIRD dosimetry has been successfully used in 131I-metaiodobenzylguanidine therapy and radioimmunotherapy (RIT) (5,6). More sophisticated techniques beyond the macrodosimetry of MIRD have evolved over the years, and new methods such as patient-specific Monte Carlo simulation and dose-point kernel convolution dosimetry have been described (7–9).
We report the experience with dosimetry-guided RAI therapy in the management of DTC patients. This report is a retrospective analysis of combined BM and tumor dosimetry application in clinical practice and addresses the safety and efficacy of high-dose 131I administration.
MATERIALS AND METHODS
One hundred twenty-four patients with diagnosis of DTC underwent 187 RAI surveys and dosimetry in hypothyroid condition for evaluation or treatment of their metastatic disease between 1986 and 2001. Demographics, distribution of iodine-avid metastases, administered 131I activity, radiation dose to critical organs (red BM, lungs) and to target metastases, BM toxicity, response to treatment, and outcome were studied.
RAI Survey and Dosimetric Data Collection
All patients were placed on a thyroid hormone withdrawal protocol to achieve a target thyroid-stimulating hormone (TSH) level of 30 mU/L. On the first day of dosimetric studies, the patients were intravenously administered 150–400 MBq 131I, and whole-body images as well as patient-specific spot views were obtained immediately after injection. Imaging continued at daily intervals up to 4–5 d. Dosimetry was performed using MIRD methodology.
Activity determinations were done using a region-of-interest (ROI) technique. Individual ROIs were drawn for whole body, organ of interest, and metastatic targets on both anterior and posterior projections. Calculations were based on the geometric mean of anterior and posterior counts. No additional attenuation or scatter correction was applied. Blood activity measurements were performed to validate the ROI technique for whole-body activity determinations. Time-activity curves were then generated and, using regression analysis, effective half-life and residence time calculations were made. MIRDOSE3 software was used to determine the dose estimates to critical organs and metastatic sites. Standard S values were applied for BM and lung dose estimations. For tumor dose estimations, conventional imaging modalities (ultrasound, CT, MRI) were used to measure target mass (volume). Formulas used in dosimetric calculations are given below.
Basic Formulas
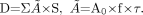
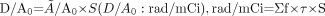 , where D = absorbed dose, Ã = cumulated activity, A0 = administered activity, S = S value, τ = residence time, and f = maximum uptake in source region.
, where D = absorbed dose, Ã = cumulated activity, A0 = administered activity, S = S value, τ = residence time, and f = maximum uptake in source region.
Tumor Dose Formula
When calculating radiation absorbed dose for localized targets (remnant, lymph node metastases, recurrent or metastatic tumor masses), the tumor dose formula is used. S values for tumor dose calculations were approximated from the spheroid models. A spheroid of matching size or weight was used for this purpose.
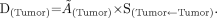
BM Dose Formula
When calculating radiation absorbed dose for BM (red marrow), the BM dose formula is used.
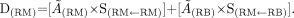
When the target mass did not have a tabulated S value in MIRDOSE3 software, corrected S values were used:
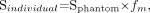 with
with
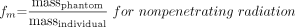 and
and

RAI Treatment Planning
The term “largest safe dose” refers to the 131I administered activity estimated to deliver limiting absorbed doses to BM or lungs. The maximum administered activity was limited with radiation doses of 3 Gy to the BM or 30 Gy to the lungs. Administration of 3 Gy to the red marrow was chosen as an acceptable risk because it corresponds to the lethal dose (LD5/5) of the external radiation therapy, which indicates a 5% risk of severe damage within 5 y to the blood-forming system in the case of the BM. Administration of 30 Gy is the LD5/5 of lungs to develop radiation-induced fibrosis.
A risk-adapted approach is defined as individual determination of the risk-to-benefit ratio in the process of treatment planning. RAI treatment intent was cure when the calculated dose to all known metastases was ≥100 Gy. When dosimetry results indicated that this dose level in the metastases could not be attained, the patients received reduced-dose RAI therapy with a palliative intent. The patients who were treated previously with RAI, or showed impaired BM function and who had significant comorbidities, also received a reduced dose. RAI treatment was deferred if no curative or palliative outcome could be foreseen.
Treatment doses of 131I were administered intravenously over 15–30 min using a lead-shielded perfusor with a secure intravenous access system.
Stem Cell Procurement
BM stimulation with granulocyte colony-stimulating factor followed by stem cell separation was performed in selected cases in collaboration with the Department of Medicine, Division of Transfusion Medicine. Stem cell preparations were stored for possible later use in case of permanent BM failure inflicted by high-dose RAI treatment.
Posttreatment Monitoring
Complete blood counts were monitored twice a week for 6 consecutive weeks. Patients with critical BM suppression (<20 platelets/nL; <1 white blood cell/nL) were admitted to the hospital for indicated treatments.
RESULTS
One hundred twenty-four patients underwent 187 RAI surveys and dosimetry evaluations over a period of 15 y. Patient and tumor characteristics are given in Tables 1 and 2. Forty-one patients (83 RAI survey or dosimetric studies) did not receive 131I treatments after dosimetry because it was not clinically indicated. Specific reasons for no treatment actions are given in Table 3.
Patient and Tumor Characteristics in 104 Patients Who Underwent Dosimetry
Tumor Localization in 95 Patients with Proven Tumor or Metastases at Time of Dosimetry
Reasons for No-Treatment Action in 41 Patients (83 Dosimetries)
Many of the patients underwent ablative RAI therapy before dosimetry, which was done in the case of recurrent or metastatic disease. Eighty-three patients received 104 treatments, of which 13 were given for postsurgical ablation of thyroid remnants, 41 were given with curative intent, and 50 were for palliation.
The dose-limiting organ was BM in 19 of 41 treatments (46%) and lungs in 4 of 41 treatments (9.8%). In the remaining 18 treatments (44%), the therapeutic endpoint of achieving a dose to the metastases of ≥100 Gy was reached delivering <3-Gy BM or <30-Gy lung limiting doses.
The administered activity delivering a 3-Gy BM dose ranged from 7.4 to 37.9 GBq (200–1,040 mCi; mean, 22.1 GBq [597 mCi]). The calculated doses to metastases ranged from 100 to >1,000 Gy.
Outcome of Patients Who Underwent Salvage Treatment
Thirty-two patients underwent at least 1 curative-intent RAI treatment (Table 4). Post-RAI treatment survival in this group ranged from 0.6 to 10.9 y (mean, 4.4 y). Six patients (19%) died of disease, and 4 patients (13%) died of other causes (bronchial asthma, suicide caused by depression, incarcerated hernia, sudden cardiac event) during the follow-up. Twelve patients (38%) showed decreased thyroglobulin (Tg) levels of <1 ng/mL after RAI treatment. Nine of these 12 patients maintained their Tg level at or <1 ng/mL over a mean follow-up of 4.3 y. In 4 patients (13%), Tg levels remained stable and <30 ng/mL over a mean follow-up of 5.6 y. Tg levels before and after therapy are given in Table 4.
Thirty-Two Patients Who Underwent at Least 1 Salvage (Curative-Intent) 131I Treatment
Mild-to-moderate xerostomia was the most common side effect among the patients who underwent curative-intent therapy. One patient, first treated by risk-adapted RIT with curative intent, received 5 treatments over a 10-y period with a cumulated activity of 94.7 GBq (2.6 Ci). All metastases (bone) were eradicated at the first therapy (Tg, <1 ng/mL). However, new metastatic sites were identified 2 y after the initial treatment. The patient underwent a second therapy with curative intent. Unfortunately, a complete response was not obtained with the second treatment. Therefore, all further therapies were given with palliative intent. This patient later developed a secondary cancer and died as a result of a pathologic hip fracture secondary to metastatic disease.
BM Toxicity
All patients (24 curative therapies and 1 palliative therapy) who received 131I doses delivering 3 Gy to the BM developed transient BM depression. BM depression reached its nadir at approximately 3–5 wk after the RAI treatment and manifested with thrombocytopenia followed by leukopenia. A spontaneous complete recovery was observed within the next 3–5 wk (Figs. 1 and 2). Four patients (2 of whom received a <3-Gy BM dose but had pretherapeutic impaired BM function) required admission to the hospital; 2 of them received transfusion of platelets and red blood cells for pancytopenia. No permanent BM failure was observed and none of the patients required stem cell treatment for recovery of their BM.
Course of platelet count after 131I therapy in 6 patients with different BM doses. Note nadir at 3–5 wk and total recovery within following 3–4 wk.
Course of white blood cell count after 131I therapy in 6 patients with different BM doses. Note nadir at 4–6 wk and total recovery within following 3–4 wk.
Lung Function
None of the patients in whom lung was the dose-limiting organ showed symptoms of impaired lung function. Pulmonary function tests were not performed because they were not clinically indicated. A patient who had 3 prior RAI treatments at another institution, presenting with pulmonary fibrosis, was not selected for further RAI treatment.
DISCUSSION
Despite the generally good prognosis in most cases, approximately 10% of the patients with DTC, some with an unexpectedly aggressive clinical course, die of their disease. The indications and appropriate use of available treatment options (i.e., surgery, RAI treatment, and thyroid hormone) have been subjects of controversy for many decades. Because of the protracted course of most thyroid carcinoma cases, it is extremely difficult to design a prospective randomized clinical trial evaluating the efficacy of RAI treatment. Large retrospective series have clearly demonstrated a significant outcome benefit for both ablation and therapy with RAI (10). The early results of the National Thyroid Cancer Treatment Cooperative Study confirmed that postoperative RAI treatment was associated with improved cancer-specific mortality rates and disease progression in both papillary and follicular cancer (11).
Currently, RAI therapy in DTC is performed by either administering an empiric fixed dose or using dosimetry-guided techniques. However, the clinical merits of dosimetry-guided RAI therapy have been clearly demonstrated in the literature (12–14). Because of the technical and logistic difficulties, most centers have adapted the fixed-dose technique using 3.7–7.4 GBq (100–200 mCi) 131I. A dosimetric approach in thyroid cancer treatment was first introduced by Benua et al. (2) in the early 1960s and has been successfully used in the management of DTC patients. The rationale of using the highest possible dose is based on the radiobiologic fact that the radiation treatment efficacy is directly related to the radiation dose delivered. The dose-limiting toxicity of RAI treatment is mainly in the BM. Lungs bearing diffuse metastases and salivary glands may also receive high doses and be at risk for expressing radiation-related effects. Therapeutic dose levels in metastatic targets may be difficult to achieve because of intolerable levels of radiation exposures in critical tissues, including but not limited to BM. On the basis of the Memorial Sloan-Kettering Cancer Center experience, it has been accepted that the activity that delivers 2 Gy to BM with a whole-body retention of <4.44 GBq (<120 mCi) at 48 h does not result in permanent BM suppression; using these guidelines, activities as high as 25.9 GBq (700 mCi) have been given safely (3). The MIRD technique, a more advanced methodology in dosimetry, has been adapted by many centers (15). We have developed a dosimetric methodology based on the MIRD technique (16) and have demonstrated that the BM limiting dose can be safely increased to 3 Gy with no permanent marrow suppression. This allowed us to administer doses as high as 38.5 GBq (1,040 mCi) at 1 time. It has been shown that the chance and length of survival are increased in patients who can be freed of their metastases by RAI treatment (17). Administration of initial high-dose RAI has several therapeutic advantages over multiple or fractionated, limited dose therapies.
There is ample evidence that the initial RAI treatment (the first strike) has the highest therapeutic effect. This observation is mainly attributed to the subsequent oncobiologic changes in the thyroid cancer cells and intratumoral biokinetic alterations resulting in diminished RAI uptake or organification. It is also commonly accepted that repeated RAI treatments in metastatic DTC with lower doses are less effective. This is mainly due to the fact that DTC is a slow-growing tumor and sublethal doses of RAI may allow adequate time for the surviving cell populations to regrow and repair the radiation damage.
Dosimetric calculations assume a homogeneous dose distribution throughout a target lesion, which is often not true in reality. The reported cytolethal doses of RAI for normal and neoplastic thyroid tissue show significant variations. Ablation of a normal thyroid tissue or an autonomous nodule was reported to require 300 Gy (18). It has also been shown in an experimental model that the neoplastic thyroid epithelial cell lines are approximately 13% more sensitive to external beam radiation than their nonneoplastic counterparts (19). One could then deduce that a dose of approximately 250 Gy is required for a tumoricidal effect in a metastatic focus. Conflicting response rates have been reported in the literature with regard to the dose-response relationship. Maxon (20) reported a favorable response in lymph node metastases with an 80-Gy dose, whereas Flower et al. (21) reported an inadequate response with 120 Gy. We observed complete responses with tumor doses ranging from 100 to 150 Gy. We also have observed a skeletal metastatic focus not responding to 480 Gy. These variable responses to treatment are due, in part, to the inhomogeneity in the RAI distribution within the metastatic deposit. Most tumors show various degrees of differentiation within. As such, different parts of a metastatic lesion may differ in their ability to concentrate RAI. Damage on the Na/I-symporter or the iodine organification system of surviving tumor cells by prior RAI treatment is certainly another important factor contributing to the nonhomogeneous distribution of RAI in repeated therapies.
The tumor clusters that are not RAI avid are destroyed by the cross-fire effect. The relative resistance of skeletal metastatic lesions is explained by attenuation of the absorbed dose due to the interference of osseous microstructures. By maximizing the administered dose, one has a better chance of overcoming the anatomic and physiologic obstacles of nonhomogeneous distribution.
The potential impact of the stunning effect on the efficacy of RAI treatment has been a subject of controversy clinically. Furthermore, the stunning effect might also alter the projected dose estimates. Both issues require more experimental and clinical data for a rational discussion. In our patients, no stunning effect could be objectively demonstrated. Additionally, no decrease in thyroid uptake was seen after dosimetry with a 370-MBq test activity (with estimated absorbed doses as high as 50–100 Gy to the thyroid remnants).
Stem cell procurement may offer additional safety for patients undergoing high-dose (salvage) therapy with RAI. Although none of the patients in our series required stem cell treatment after RAI, such supportive backup might be appropriate for more aggressive salvage therapies.
The risk of leukemia from high-dose RAI treatment has also been a subject of controversy. The incidence of leukemia appears to be related to the cumulated administered activity or, more exactly, to the cumulated red BM dose rather than to a single RAI treatment dose (especially if the activity is <18.5 GBq [<500 mCi]). An incidence of acute myelocytic leukemia of 1 or 2 per 100,000 per year has been reported after a mean cumulated activity of 40.7 GBq (1,100 mCi), equaling a 3.2-Gy red BM dose. The mean latency was 42 mo (22). To date, we have not observed a single case of leukemia in our patients. One could even speculate that a longer recovery period for the BM after RAI treatment might promote cell repair mechanisms and lower the incidence of leukemia. More data and longer follow-up are needed to answer these questions more conclusively.
In the early post-RAI treatment follow-up (the first 2 mo), the possibility of severe hematologic complications requires intense cooperation with the hematologist and the primary care physician. The patient’s strict compliance to monitoring of the blood count is also crucial.
CONCLUSION
Dosimetry-guided high-dose (BM absorbed dose up to 3 Gy) RAI therapy is a safe approach in the treatment of patients with DTC. This approach might also reduce the cumulated administered activity compared with the repeated, limited dose schedules (3.7–7.5 GBq [100–200 mCi] every 3 or 6 mo) and, hence, may reduce the delivery of unnecessary radiation dose to marrow and other tissues. A risk-benefit assessment before high-dose RAI therapy is essential. The therapeutic benefits certainly outweigh the cost and labor associated with radiation protection measures and potential stem cell procurement applications. Decreased total hospital stay and pretreatment preparation period (especially when a thyroid hormone withdrawal protocol is applied) favorably affect the quality of life in patients with DTC.
Footnotes
Received Mar. 25, 2002; revision accepted Sep. 25, 2002.
For correspondence or reprints contact: Seza A. Gulec, MD, John Wayne Cancer Institute, 2200 Santa Monica Blvd., Santa Monica, CA 90404.
E-mail: gulecs{at}jwci.org








