Mankoff et al. (1) address the application of PET for monitoring neoadjuvant chemotherapy in locally advanced breast cancer (LABC) in this issue of The Journal of Nuclear Medicine.
An important advantage of primary chemotherapy is that it can increase the rate of breast-conserving surgery by preoperatively reducing tumor volume (2). Because of the good results of this procedure, chemotherapy before definitive surgical therapy (neoadjuvant, primary, or induction chemotherapy) has also been applied to the treatment of lower-stage breast cancer. Recent trials reported clinical response rates greater than 70%, but only in a small fraction of patients has a macroscopic complete response to therapy been achieved (3). Patients who have a macroscopic complete response also have a significantly better prognosis than patients presenting with residual tumor after completion of chemotherapy (4). Patients with minimal residual disease have significantly higher disease-free and overall survival rates than do patients with gross residual disease. Partial or complete regression proven by histopathologic tissue analysis is achieved in only 20%–30% of patients (5). The clinical response thus does not necessarily reflect histopathologic response, which necessitates evaluation and tumor markers to determine the therapeutic effect until definitive breast surgery is performed. Considering the side effects of primary chemotherapy, there is a need for early therapy monitoring to identify nonresponding patients. Imaging procedures such as mammography, ultrasonography, and MRI have been used for evaluating tumor size in relation to response to therapy. One of the drawbacks of anatomic imaging, however, is the delay between initiation of therapy and tumor shrinkage. In addition, these procedures do not allow differentiation between viable tumor tissue and fibrotic scar tissue (6). Although evaluation and tumor markers might be useful for this purpose, they have not yet been applied in larger patient populations (7).
For these reasons, nuclear medicine procedures offer opportunities for the evaluation of the success of chemotherapy. In addition to the use of 99mTc-isonitriles (99mTc-sestamibi) for evaluation of therapy success (8), PET represents a useful method. One of the factors that may influence response to systemic chemotherapy is tumor perfusion (9), which can be evaluated by PET using 15O-water (10) and 99mTc-sestamibi with traditional single-photon imaging (8,11). Good perfusion is crucial for the delivery of chemotherapy to the tumor cell. Tumors with low perfusion may be hypoxic, and hypoxia has been related to aggressive tumor behavior and poor response to chemotherapy (12).
A second procedure offered by nuclear medicine is 18F-FDG PET, which has previously been shown to be beneficial in monitoring the response to chemotherapy (13–16). The experiences with 18F-FDG PET are encouraging but somewhat controversial. Despite these drawbacks, 18F-FDG PET allows prediction of the response to chemotherapy in LABC even shortly after the onset of therapy. Thus, 18F-FDG PET is expected to be useful for reducing the costs of cytotoxic therapy and the unnecessary side effects of chemotherapy that is not useful. These economic and clinical advantages will certainly outweigh the expense of PET. A prerequisite for monitoring therapy response is, of course, quantification of PET data.
QUANTITATION OF PET
Most PET researchers use standardized uptake values (SUVs) for quantification of PET. Because the tumor shrinks during response, the placement of regions of interest (ROIs) is crucial. It has been proposed that circular ROIs with a diameter of 1.5 cm should be placed (14). This procedure has been chosen to reduce partial-volume effects, which play a substantial role if a ROI is placed visually around the entire tumor and tumor size changes after the baseline study (14).
Smith et al. (15) have used a cumbersome protocol in performing a time-consuming dynamic imaging study and collecting sequential arterial blood samples. The influx constant was calculated for each voxel of the image, resulting in a parametric map of 18F-FDG uptake. The dose-to-uptake ratio (DUR) is also calculated for each voxel from the final frame of the dynamic data using the following equation:
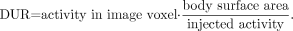
Mankoff et al. (7) calculated the metabolic rate of 18F-FDG (MRFDG) using the tracer flux constant Ki (mL/min/g), where the metabolic rate was calculated as follows:
 thus resulting in units of micromoles per minute per 100 g.
thus resulting in units of micromoles per minute per 100 g.
PET perfusion estimates using 15O were calculated according to the method of Wilson et al. (10) using a 1-compartment model, resulting in units of milliliters per minute per gram for tumor and normal tissue. These perfusion data were combined with MRFDG.
All quantitative 18F-FDG protocols require constant glucose levels; thus, the glucose level has to be determined before injection. The follow-up data also have to be normalized to the blood glucose level.
Most studies have focused on serial PET scans. Mankoff et al. (7) attempted to predict the outcome by a baseline study alone. However, they combined both 15O and 18F-FDG PET in the study.
WHICH PROTOCOL WHEN?
Bender et al. (17) have shown that—at least in nonresectable liver metastases of colorectal cancer—early identification of nonresponders is possible. PET studies conducted before and 72 h after a single infusion of 5-fluorouracil and folinic acid showed a 22% decrease in the SUVs early after the onset of therapy. Smith et al. (15) obtained 4 PET scans: at baseline, before the second and fifth doses of chemotherapy, and shortly before surgical excision of the primary tumor and axillary lymph nodes. Schelling et al. (14) used a protocol including a PET scan 10 d after the first course and 9 d after the second course of chemotherapy, in addition to the baseline study. Mankoff et al. (1) added to the previous protocol a follow-up PET scan (15O water and 18F-FDG) after 2 mo of chemotherapy.
The results of these studies may be summarized as follows: Using the baseline 18F-FDG and 15O-water PET only, Mankoff et al. (7) identified a statistically significant trend for patients with pretherapy high MRFDG to have a poorer response to therapy. A low ratio of MRFDG to blood flow (μmol/min/100 g per mL/min/100 g) was the best predictor of microscopic complete response. The data were extended by a second PET examination after 2 mo of chemotherapy (1), and responders showed a greater decline in MRFDG than nonresponders. Responders had a decline in tumor blood flow, whereas nonresponders had an increase, the difference being statistically significant. The change in blood flow after 2 mo of therapy predicted disease-free and overall survival (1). Interestingly, in this study (1) the difference between responders and nonresponders—with respect to 18F-FDG metabolism—was not significant. In contrast, other researchers have concluded that the decrease in 18F-FDG uptake is a marker of tumor response. Smith et al. (15) performed serial PET studies using 18F-FDG uptake (DUR) only and concluded that 18F-FDG PET imaging of primary and metastatic breast cancer after a single course of chemotherapy may be of value in predicting pathologic treatment response (sensitivity of 90%, specificity of 74%). However, in metastatic lesions this effect could not be observed. In contrast to Mankoff et al. (7), Smith et al. found that the mean pretreatment DUR values (18F-FDG) of the lesions that achieved a microscopic complete pathologic response were significantly higher than those from less responsive lesions. Schelling et al. (14) also correctly identified responders by follow-up PET, with a sensitivity of 100% and a specificity of 85%. A threshold was set by an SUV decrease below 55% of the baseline scan. At this threshold, histopathologic response could be predicted with an accuracy of 88% and 91%, respectively, after the first and second courses of therapy.
These data refer to chemotherapy only. Dehdashti et al. (18) published PET data for patients receiving antiestrogen therapy (16α-18F-fluoro-17β-estradiol [FES]), and these data were correlated with up to 24 mo of follow-up data. None of the responders had a clinical flare reaction, but all demonstrated metabolic flare, with an increase in tumor SUVs for 18F-FDG. No flare was evident in the nonresponders. The amount of estrogen receptor located by tamoxifen (FES) was greater in responders than in nonresponders. These data indicate that the possibility that 18F-FDG PET will show metabolic flare early after the administration of tamoxifen therapy has to be kept in mind. The flare phenomenon has never been observed after chemotherapy.
Another problem that should be addressed is secondary effects such as neovascularization and inflammatory cell infiltration, which may lead to changes in tumor blood flow and may mask tumor response. However, these changes will be observed mainly after radiation therapy and not after chemotherapy. Any apparent influence should be confirmed by animal experiments.
CONCLUSION
In a clinical setting, 18F-FDG PET has to be recommended for predicting response to chemotherapy in LABC. Cumbersome techniques such as the combination of 18F-FDG with 15O-water imaging may be restricted to research. The data published by Schelling et al. (14) allow the conclusion that the estimation of SUVs (in defined circular ROIs) should be recommended for clinical purposes. These SUVs will not be hampered by partial-volume and tumor shrinkage effects. The SUVs of baseline and follow-up investigations should be normalized to the blood glucose level. With the increasing availability of clinical PET, the use of this powerful technology for monitoring primary chemotherapy is expected to become routine.
Footnotes
Received Jul. 21, 2003; revision accepted Jul. 24, 2003.
For correspondence or reprints contact: Hans J. Biersack, MD, Department of Nuclear Medicine, University Hospital Bonn, Sigmund-Freud-Strasse 25, D 53127 Bonn, Germany.
E-mail: hans-juergen.biersack{at}meb.uni-bonn.de






