Abstract
Ciprofloxacin, a quinolone antibiotic drug, binds to DNA topoisomerase IV and DNA gyrase of various bacteria. Thus ciprofloxacin labeled with 99mTc could potentially act as a specific marker allowing discrimination between infection and sterile inflammation. We evaluated these properties on a rabbit model of prosthetic joint infection previously validated. We compared the images obtained in 2 groups of animals: rabbits with infected (G1; n = 6) and uninfected (G2; n = 7) prosthesis. Methods: Partial right-knee arthroplasty was performed on 13 New Zealand White female rabbits, with a tibial silicone-elastomer implant fitting into the intramedullary canal of the tibia. After the surgical wound was closed, 107 cfu of a clinical strain of methicillin-susceptible Staphylococcus aureus were injected into the joint in G1 rabbits. G2 rabbits were injected with sterile saline. No antibiotic therapy was given to the animals. 99mTc-ciprofloxacin planar imaging was performed on days 5, 12, and 19 after surgery, and after 3 mo in 1 uninfected rabbit. Images were obtained 1, 4, and 24 h after injection (147 ± 13 MBq). Results: In G1, increased right knee 99mTc-ciprofloxacin uptake was observed in 3 of 5 rabbits on day 5, and in all cases on days 12 and 19. Killing of the animals revealed purulent arthritis, osteitis, and tibial myelitis. In G2, significant right-knee uptake was found on days 12 and 19 in 5 of 6 rabbits, and after 3 mo in 1; all sets of images were negative in 1 animal. Bacteriologic studies after the animals were killed were negative in G2. Mean right/left knee uptake ratios on day 19 (4-h images) were 1.8 ± 0.4 in G1 versus 1.4 ± 0.3 in G2 (not significant). Late images did not discriminate between infected and uninfected arthroplasty. Conclusion: Results of 99mTc-ciprofloxacin imaging in rabbits with infected/uninfected knee prosthesis suggest good sensitivity but lack of specificity for the detection of S. aureus infection.
The discrimination between bacterial infection and sterile inflammation is clinically relevant in many situations, but difficult. Especially in painful joint prosthesis, accurate diagnosis between aseptic and septic loosening is important as both the surgical management and outcome may differ depending on whether the arthroplasty loosening is infective or mechanical in origin. In the presence of infection, delayed reconstruction after antibiotic therapy during several weeks is needed in most cases (1–4). Clinical, biologic, and radiologic criteria are not sensitive or specific in cases of late-onset septic loosening (1,2). Fluid cultures after joint aspiration may also be negative in the presence of infection (5). Very recently, authors focused on the optimization of intraoperative tissue sample histologic analysis as a guide to whether a 1-stage (for aseptic loosening) or 2-stage (for septic loosening) procedure needed to be performed (1).
Radionuclide imaging has been extensively used for the preoperative diagnosis of prosthetic joint infection. But most radiopharmaceuticals proposed, such as gallium, polyclonal or monoclonal immunoglobulins, cytokines, or liposomes, in fact constitute tracers of the inflammation process. 99mTc-HMPAO leukocytes carry the major drawbacks of ex vivo blood cell manipulation, with associated infectious risk, and long duration of radiolabeling (2,6). Clearly, radiopharmaceuticals that specifically bind to a variety of bacteria would be better candidates for infection imaging (6). The first such radiopharmaceutical proposed was ciprofloxacin radiolabeled with 99mTc, which is absorbed by various bacteria (7). The results of human clinical trials in many infectious diseases, but no experimental animal data, have been reported in the literature (8–10).
Our aim was to test the ability of 99mTc-ciprofloxacin imaging to discriminate between infected and uninfected prosthetic joints, using a rabbit model of prosthetic joint infection previously validated (11).
MATERIALS AND METHODS
Test Strain
A methicillin-susceptible strain of Staphylococcus aureus (MSSA) was used for infection induction. The strain was originally isolated from a patient with an infected knee prosthesis. Its virulence was maintained by intraperitoneal injection into mice. The day before inoculation, the bacteria were suspended in trypticase soy broth and grown overnight at 37°C. The next day, the culture was diluted in phosphate buffer, pH 7.4, and serial dilutions were made in sterile saline to obtain the final inoculum.
Prosthesis Infection
Thirteen New Zealand White female rabbits weighing 2.9–3.5 kg and aged 74–120 d (except 1 animal, 6 mo old) were studied. They were housed in individual cages with a natural light–dark cycle. The experimental protocol was in keeping with French legislation on animal experimentation.
This model has been described in detail elsewhere (11). Briefly, a surgeon partially replaced the rabbit’s right knee with a tibial component using a silicone-elastomer implant (Silastic, great toe implant HP, Swanson Design; Dow Corning, Valbonne, France). The operation was performed under general anesthesia induced by intramuscular injection of ketamine (25 mg/kg of body weight) and then continuous inhalation of 1% isoflurane. The skin of the animal’s right leg was shaved 24 h before the operation. Before surgery, the skin was cleaned with an iodine solution. A longitudinal skin incision was made, and the knee was exposed. After dislocation of the tibia, the epiphysial plates were removed. The metaphysis was exposed, and the cancellous bone of the medullary cavity of the proximal metaphysis was reamed. The stem of the nail-shaped silicone implant (14 mm long) was inserted into the intramedullary canal of the tibia, with the implant head (15 × 5 mm) replacing the tibial plateus. Then the deep fascia and the skin were closed.
Just after the surgical wound was closed, 6 rabbits were infected by intraarticular injection of 0.5 mL inoculum containing 107 colony-forming units MSSA (G1 = infected group). The other 7 rabbits were injected with 1 mL sterile saline solution (G2 = uninfected group).
99mTc-Ciprofloxacin Imaging
99mTc-ciprofloxacin scintigraphy was performed 5, 12, and 19 d after surgery, except in 1 case (an uninfected rabbit who had imaging 3 mo after surgery). 99mTc-ciprofloxacin was prepared using kits manufactured in St. Bartholomew’s Hospital (London, U.K.), which comprised 2 vials: vial 1 contained 2 mg ciprofloxacin, and vial 2 contained 500 μg stannous tartrate. For radiolabeling, we strictly followed the instructions the manufacturers directly supplied to us (7–10). Sodium pertechnetate (400 MBq ± 20 MBq) was drawn up from a 99mTc generator freshly eluted (within 24 h). The contents of vial 1 were drawn up using an orange needle, 25 gauge. One milliliter of sodium chloride 0.9% was injected in vial 2 (stannous tartrate), which was shaken to dissolve dried powder. Then the sodium pertechnetate was added to vial 2, immediately followed by the contents of vial 1. After shaking, the final preparation was left to stand for 5 min. The quality control was performed with Whatman No. 1 paper chromatography (Whatman International, Maidstone, Kent, U.K.), using butanone as eluent, and with Sep-Pak (Waters Corp., Milford, MA). Then the preparation (147 ± 13 MBq) was injected intravenously (ear vein) to the animals within 1 h of the radiolabeling. The rabbits were not anaesthetized, but tied to a board before imaging. Ten-minute planar anterior and posterior views of the legs were obtained 1, 4, and 24 h after injection using a dual-head gamma camera fitted with high-resolution parallel collimators. Images with a pinhole collimator were also obtained in 4 animals. On each anterior scintigram, 3 anatomically adjusted regions of interest (ROIs) were drawn over the operated and the contralateral normal knee: 1 over the femoral part of the knee, 1 over the tibial part of the knee, and 1 over the whole knee. Mean activity (cpm) per pixel was determined in each ROI. Then the operated-to-normal-knee activity ratios (ONKR) were calculated.
Biodistribution of 99mTc-Ciprofloxacin
Blood tracer kinetics were determined in 4 animals by counting 100-μL blood aliquots (taken 5, 10, 15, 30, and 45 min and 1, 4, 12, and 24 h after injection) in a well scintillation γ-counter. In 4 G1 and 5 G2 rabbits, blood and tissue samples of organs and right and left knees were taken after the animals were killed on day 20 after surgery (24 h after 99mTc-ciprofloxacin injection). Samples were weighed, and counted in a γ-counter. Autoradiography of the knees was performed on 1 infected rabbit killed 3 h 30 min after 99mTc-ciprofloxacin injection. After being skinned, both limbs were embedded in carboxymethyl cellulose 2% and then frozen in hexane containing carboxic ice (−80°C). Sections (100 μm thick) of the limb, including prosthesis, bones, ligaments, and part of the surrounding muscles, were cut at –30°C in an LKB macrocryostat (PMV-LKB Pharmacia, Stockholm, Sweden) and collected on adhesive tapes. The sections were then exposed in a γ-imager (Charpak Instant Imager; Packard Instrument Co., Meriden, CT) during 10 h.
Microbiologic Features
In G2 animals, the exudate localized on the proximal part of the prosthesis was spread onto a blood-agar plate and incubated overnight at 37°C to confirm the absence of infection. Quantitative bacterial counts were determined in 2 G1 animals: tissue samples of the infected arthroplastic knee were isolated under aseptic conditions, weighed, then cut into small pieces, frozen in liquid nitrogen, crushed in an autopulverizer (Spex 6700; Freezer/Mill Industries, Metechen, NJ), and quantitatively cultured.
Statistical Analysis
Differences between the values for ONKR and sample cpm counts were analyzed using the Mann–Whitney U test or Wilcoxon signed rank test. The level of significance was set at P < 0.05.
Results
Labeling Control
Using Whatman paper chromatography and Sep-Pak verification of the final preparation, the purity was always >96% with the 2 techniques. This excludes the presence of radiolabeled colloids to a significant extent.
Scintigraphic Results
Two G1 and 1 G2 animals died during the study period. Scintigraphy was performed on 5 animals of each group on day 5 after surgery, on 4 animals of each group on days 12 and 19, and on 1 uninfected rabbit 3 mo after surgery.
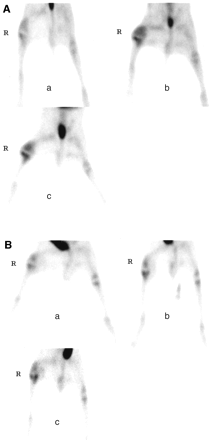
Intense renal activity was observed, because 99mTc-ciprofloxacin is excreted through this route to the bladder. Faint liver activity was observed on all images. No activity was noted in bone marrow or in normal bone, muscle, or soft tissue. However, 99mTc-ciprofloxacin uptake was noted in growing epiphysial disk cartilage; this was very intense in young animals and decreased with age (mild uptake in a 1-y-old rabbit). In infected rabbits (G1), visual analysis revealed significant increased 99mTc-ciprofloxacin uptake in the operated knee compared with the nonoperated knee (“positive scan”) in 3 (60%) of 5 animals on day 5 after surgery, and in 4 (100%) of 4 animals on days 12 and 19. In uninfected rabbits (G2), all scans were negative on day 5 after surgery, but significant 99mTc-ciprofloxacin uptake was observed in the operated knee in 3 (75%) of 4 animals on days 12 and 19, and also after 3 mo in 1 animal. In animals with positive scans from the 2 groups, 99mTc-ciprofloxacin uptake was seen in the tibial and femoral areas of the operated knee. The area of uptake expanded with time after surgery, from the tibial plateus on day 5, to the whole tibial metaphysis, and then to the soft tissues in the femoral area (Fig. 1). Tibial expansion seemed more evident in G1 rabbits, even reaching the upper diaphysis, as seen on the autoradiography (Fig. 2). Mean values of ONKR in articular ROIs (4-h images) increased with time after surgery in the 2 groups: from 1.12 ± 0.14 (day 5) to 1.82 ± 0.46 (day 19) in infected animals; and from 1.05 ± 0.23 (day 5) to 1.43 ± 0.29 (day 19) in uninfected rabbits. ONKRs were never significantly different between the 2 groups (Fig. 3). By visual analysis, no significant difference was observed between 1-, 4-, and 24-h images in any of the animals (Fig. 4). ONKR values in articular ROIs slightly increased on 24-h images in infected animals on days 12 and 19 after surgery, while they remained stable (but never decreased) in uninfected rabbits. Mean 1-, 4-, and 24-h ONKRs (regardless of time after surgery) in the 2 groups are shown in Figure 5.
99mTc-ciprofloxacin scintigraphy (images obtained 4 h after injection). (A) Infected rabbit: increasing and expanding 99mTc-ciprofloxacin uptake in right infected prosthetic knee (a) 5 d, (b) 12 d, and (c) 19 d after surgery. (B) Uninfected rabbit: significant, increasing 99mTc-ciprofloxacin uptake in right uninfected prosthetic knee (a) 5 d, (b) 12 d, and (c) 19 d after surgery.
Autoradiography of knee in infected rabbit. (A) Normal (nonoperated) knee: (a) epiphysial disk cartilage, (b) articular cartilage. (B) Infected prosthetic knee: (c) muscle, (d) articular cartilage, (e) tibial prosthesis, (f) tibial bone. (C) Pus.
Operated-to-normal-knee activity ratios (ONKR) in G1 (infected) and G2 (uninfected) rabbits according to time after knee arthroplasty.
99mTc-ciprofloxacin images of uninfected rabbit (A) 1 h, (B) 4 h, and (C) 24 h after 99mTc-ciprofloxacin injection. No decrease in 99mTc-ciprofloxacin uptake is seen in left prosthetic uninfected knee on 24-h images.
Mean operated-to-normal-knee activity ratios (ONKR) in G1 (infected) and G2 (uninfected) rabbits according to time after injection of 99mTc-ciprofloxacin (regardless of time after surgery).
Blood Tracer Kinetics
The half-life of 99mTc-ciprofloxacin in the blood pool was 55 ± 17 min (range, 30–70 min). Residual activity was 31% ± 1% of initial blood activity at 4 h, 21% ± 2% at 12 h, and 17% ± 5% at 24 h.
Microbiologic Features
The prosthetic knee exudates of G2 animals were sterile. Bacterial counts in G1 animals on day 20 after infection were 10+4.10–10+6.67 in tibial bone marrow, tibial and femoral cortical bone, inferior thigh muscle, femoral articular cartilage, and pus.
Biodistributions
Tissue uptake of 99mTc-ciprofloxacin (activity per gram of tissue/injected dose per gram of rabbit) was determined for thigh muscle, femoral articular cartilage, upper tibial bone, and upper tibial bone marrow of both knees in each animal, as well as for pus and articular fluid of operated knees in G1 (n = 4) and G2 (n = 5) rabbits, respectively (Table 1). In infected prosthetic knees, the uptake values of all tissues studied were at least twice the values obtained in contralateral normal knees. In uninfected prosthetic knees, the uptake values were comparable with those of normal contralateral knees, except in femoral articular cartilage and thigh muscle, for which the right and left knee uptake ratios reached 3.01 ± 0.56 and 1.45 ± 0.87, respectively. Uptake values in pus (G1 animals) and in inflammatory articular fluid (G2 animals) were comparable (56 ± 23 vs. 55% ± 24%, not significant). Articular fluid of normal knees could not be studied because the volumes obtained were too small.
Biodistributions of 99mTc-Ciprofloxacin in Prosthetic and Normal Contralateral Knees of Infected (G1) and Uninfected (G2) Rabbits
Autoradiography
As seen in Figure 2, there is intense 99mTc-ciprofloxacin uptake in epiphysial disk cartilage of normal and prosthetic knees. Very intense uptake is observed in femoral articular cartilage and tibial cortical bone of infected knee, highlighting the outlines of the inferior femoral epiphysis and tibial diaphysis. Diffuse increased 99mTc-ciprofloxacin uptake is also seen in the whole articular cavity of the infected knee (pus), compared with the contralateral normal knee.
Discussion
Orthopedic replacement is a very common operation worldwide, and foreign-body infections are a major cause of morbidity and implant failure. The overall incidence of bacterial joint infections complicating primary arthroplasty is 0.5%–2% (2). Most prosthesis infections are caused by perioperative contamination, mainly by S. aureus or Staphylococcus epidermidis. The prosthesis can also be infected via the bloodstream (2). Early diagnosis and appropriate management, usually combining debridement and prolonged antibiotic therapy, are essential to preserve joint function (1–4). But diagnosis, particularly discrimination between infected and aseptic mechanical loosening, remains very difficult. Radiographic abnormalities appear late and cannot distinguish between early mechanical loosening and low-grade sepsis. Clinical and biologic data (1,2) and MRI (12) cannot always discriminate between infection and inflammation accompanying mechanical loosening. Aspiration of joint fluid can sometimes identify the infecting organism, but the cultures can also be negative in the presence of infection: Its sensitivity for infection is low, approximately 50% (5).
Most radiopharmaceuticals proposed for scintigraphic infection imaging, such as 67Ga, 111In-oxin, polyclonal or monoclonal antigranulocytes and antibodies, cytokines, and liposomes, in fact constitute tracers of the inflammation process (2,6). Late 99mTc-HMPAO leukocyte uptake appears to be specific for the infectious process, but this tracer carries the major drawbacks of ex vivo blood cell manipulation, with associated infectious risk, and long duration of radiolabeling (13). Tracers that could specifically target bacteria would be more appropriate in this situation. Fluoroquinolones are antimicrobial agents that act by inhibiting homologous type II topoisomerases, DNA gyrase, and DNA topoisomerase ΙV (14,15). These enzymes control DNA topology and are vital for chromosome function and replication. 99mTc-ciprofloxacin, which is ciprofloxacin labeled with 99mTc, has been reported to be taken up by various bacteria, and appears to be a good candidate for in vivo discrimination between infection and inflammation in humans (7–10). No experimental assessment of 99mTc-ciprofloxacin imaging has previously been reported in an animal model.
In accordance with previous clinical human studies, our results suggest good sensitivity of 99mTc-ciprofloxacin imaging for the detection of MSSA prosthetic joint infection, with tracer accumulation in all infected tissues close to the prosthesis (8–10). However, significant 99mTc-ciprofloxacin uptake was also observed in most uninfected joints on scintigraphic images, increasing with time after surgery, and persisting 24 h after injection (late images did not allow discrimination between infected and uninfected joints). Such data are discordant with those of previous clinical studies, which reported good specificity of 99mTc-ciprofloxacin imaging (8–10). Reasons for this discordance may include the difficulty of designing clinical studies in humans that can evaluate the specificity of imaging, the heterogeneity of the diseases studied, and the absence of control groups. The use of an animal model is of interest because it allows direct comparison of infected and uninfected joints, with all other parameters being comparable in the 2 groups. Also, the model of prosthetic joint infection used in this study is easily reproducible and closely mimics acute postoperative infection in humans, as shown previously by histologic examinations (11). In uninfected animals, a nonspecific inflammatory process caused by the presence of the prosthesis has been observed in the tissues close to the device, as in humans, especially when the prosthesis was loosened (16,17).
Our data are concordant with those of previous quantitative autoradiographic studies in the same animal model, which showed similar concentrations of 14C-sparfloxacin in the artificial joint space of infected and uninfected animals (16). Recently, Welling et al. (18,19) reported similar accumulation of 99mTc-ciprofloxacin in foci of infection and in sterile inflammatory lesions induced in the thigh muscle of mice. Such accumulation could be caused by 99mTc-ciprofloxacin penetration in inflammatory cells since ciprofloxacin, like other fluoroquinolones, is concentrated in and transported by human macrophages, monocytes, and neutrophils (5- to 10-fold) (20–25). 99mTc-ciprofloxacin penetration into fibrin, an essential component of the inflammatory response, could also be supposed since sparfloxacin, pefloxacin, and temafloxacin were previously found to be highly concentrated, with homogeneous distribution, in fibrin cardiac vegetations (26,27).
Many studies have also reported an inhibitory effect of quinolones on chondroblasts, capsular fibroblasts, osteoblasts, and endothelial cells (28–32). Adverse effects on developing cartilage and ligaments were reported, leading to the contraindication of quinolones in children and pregnant women (33–35). A recent study by Huddleston et al. (36) also showed an inhibitory effect of ciprofloxacin on experimental fracture healing, with decreased callus strength, decreased size and number of chondrocytes at the endochondral ossification front, and abnormalities of trabecular bone formation. The action of ciprofloxacin as an inhibitor of a mammalian analog for DNA gyrase has been proposed as a possible mechanism (37). Fluoroquinolone uptake by cultured human epithelial cells and specific binding to human free DNA have also been previously shown (38,39).
Therefore, 2 main hypotheses can be proposed to explain nonspecific 99mTc-ciprofloxacin accumulation in inflamed aseptic articular fluid, in growing epiphysial disk cartilage, and in injured femoral articular cartilage of prosthetic knees (infected or uninfected). One could be increased blood-pool activity, especially on early images. Also, the persistence of 99mTc-ciprofloxacin uptake on 24-h images may indicate another, more specific mechanism for tracer accumulation in uninfected knees. As ciprofloxacin binds to DNA gyrase in many cells, it could be expected that leukocytes and chondrocytes would be labeled in this way with 99mTc-ciprofloxacin.
In terms of size of the study group (6 animals in G1, 7 in G2), this study was similar to other studies in animal models of bacterial infections, especially pharmacologic studies with antibiotic drugs (16). The rather small number of animals used in these studies is partly caused by ethical considerations, because of the surgery and infection inflicted on the animals. Another reason is the good reproducibility of the model, as previously shown (11). For each group we compared 99mTc-ciprofloxacin uptake in operated knees versus nonoperated knees; thus, each animal was its own control. Consequently, our results show good reproducibility in each group, with acceptable SDs (approximately 20%) for the ONKRs calculated on the images. Also, the increased 99mTc-ciprofloxacin uptake with time after surgery in uninfected prosthetic knees was clearly observable, since the animals were compared with themselves at the different times (probably because the inflammatory process and periprosthetic tissue injury both increased with the presence of the prosthesis).
Conclusion
Our results show 99mTc-ciprofloxacin accumulation in MSSA arthroplastic joint infection, but also in uninfected prosthetic joints in rabbits. Nonspecific accumulation seems to be related to inflammation and uptake by injured tissues (especially cartilage) close to the device. According to a recent study by Welling et al. (6), some antimicrobial peptides showing more specific binding to bacteria could be better candidates to differentiate between prosthetic joint infection and aseptic mechanical loosening.
Footnotes
Received Apr. 12, 2001; revision accepted Oct. 2, 2001.
For correspondence or reprints contact: Dominique Le Guludec, MD, PhD, Service de Médecine Nucléaire, Hôpital Bichat, 46 rue Henri Huchard, 75018 Paris, France.
E-mail: dominique.leguludec{at}bch.ap-hop-paris.fr