Abstract
Myocardial viability was assessed by 99mTc-methoxyisobutylisonitrile (MIBI) SPECT and 18F-FDG PET to evaluate the prognosis and treatment strategy of patients with myocardial infarction (MI) and left ventricular (LV) dysfunction. Methods: One hundred twenty-three consecutive patients with previous MI and LV dysfunction (LV ejection fraction [EF], 35% ± 6% [mean ± SD]) who underwent 99mTc-MIBI SPECT and FDG PET were followed-up for 26 ± 10 mo (mean ± SD). Distributions of the 2 radiotracers in myocardial segments were classified into 2 patterns: myocardial perfusion–metabolism mismatch (MM) and match (M). LV EF and LV end-diastolic diameter (EDD) were measured by echocardiography at baseline, 3 mo (Pos1), and 6 mo (Pos2) after revascularization. Cardiac death, acute MI, unstable angina, and late revascularization (>3 mo) experienced by the patients during follow-up were defined as cardiac events. Results: Sixty-seven patients underwent revascularization and 56 patients were treated medically. Of the 72 patients with ≥2 MM segments, 42 underwent revascularization (group A1) and 30 were treated medically (group A2). Of the 51 patients with <2 MM segments, 25 underwent revascularization (group B1) and 26 were treated medically (group B2). The 4 groups had similar baseline characteristics and rest LV EF. After revascularization, EF (mean ± SD) increased in group A1 from 36% ± 5% to 44% ± 8% (P < 0.0001) in Pos1 and to 51% ± 9% (P < 0.0001) in Pos2. EDD (mean ± SD) decreased from 62 ± 8 mm to 56 ± 5 mm (P < 0.001) in Pos1 and to 55 ± 5 mm (P < 0.001) in Pos2. However, EF and EDD were unchanged in group B1 (P > 0.05). During the follow-up, 22 patients (17.9%) suffered from cardiac events, including 11 cardiac deaths, 4 acute MI, 6 late coronary artery bypass grafting, and 1 unstable angina pectoris. The cardiac event rate in group A2 (50%) was significantly higher than that of groups A1 (2.4%; χ2 = 23.08; P < 0.0001), B1 (12%; χ2 = 8.94; P = 0.003), and B2 (11.5%; χ2 = 9.45; P = 0.002). Conclusion: Assessment of myocardial viability using hybrid 99mTc-MIBI SPECT and FDG PET can predict the clinical outcome and is helpful to decision making in the treatment strategy of patients with MI and LV dysfunction. Revascularization can improve the LV function and clinical outcome of patients with >2 viable myocardial segments.
The fact that patients with previous myocardial infarction (MI) and left ventricular (LV) dysfunction have a marked propensity for cardiovascular morbidity and mortality is evident (1). Therefore, identification and proper management of such patients are very important not only for decreasing the mortality but also for improving the quality of life (2,3). Several studies have shown that 99mTc-methoxyisobutylisonitrile (MIBI) myocardial perfusion SPECT and 18F-FDG myocardial metabolic PET are comparable with 13N-ammonia perfusion PET and FDG metabolic PET in evaluation of myocardial viability and suitability for revascularization in patients with chronic coronary artery disease (CAD) (4–9). Previous studies using 13N-ammonia or rubidium perfusion PET and FDG metabolic PET have revealed that myocardial viability is a powerful predictor of subsequent cardiac events (10–12). Other studies have shown that improvement of LV function after revascularization is related to the extent of myocardial viability (13,14). However, the role of hybrid 99mTc-MIBI perfusion SPECT and FDG PET in evaluation of the prognosis, treatment strategy, and improvement of LV function in patients with MI and LV dysfunction remains to be clearly defined. The purpose of this study was to evaluate whether the presence or absence of myocardial viability assessed with 99mTc-MIBI SPECT and FDG PET could predict the clinical outcome of patients with previous MI and LV dysfunction and whether revascularization could improve the prognosis and LV function in such patients with viable myocardium.
MATERIALS AND METHODS
Study Population
One hundred twenty-three patients with previous MI and LV dysfunction between March 1997 and May 1998 were enrolled in this study. The inclusion criteria were as follows: patients with previous MI confirmed by history, electrocardiography, and coronary angiography; and patients with rest LV ejection fraction (EF) ≤45% determined by 2-dimensional echocardiography. Patients with recent MI (<8 wk), unstable angina, cardiomyopathy, valvular disease, previous coronary artery bypass grafting (CABG), or previous percutaneous transluminal coronary angioplasty (PTCA) were excluded. All decisions concerning the choice of treatment were made by the referring physicians and were not based on a random assignment. The ethics committee of the Cardiovascular Institute and Fu Wai Hospital, the Chinese Academy of Medical Sciences, and Peking Union Medical College approved the study protocol.
Coronary Angiography
Selective coronary angiography was performed (using Judkins’ technique) on all patients within 4 wk of radionuclide imaging studies. Two experienced observers who were unaware of the clinical data visually assessed each study. The severity of coronary artery stenosis is expressed as a percentage of luminal diameter narrowing. Significant CAD was defined as ≥50% stenosis of at least 1 of the major coronary arteries or their major branches.
FDG Metabolic PET
FDG PET was performed using a whole-body PET scanner (Chinese PET-B03; Beijing High Energy Institute, Beijing, China) with 2 bed positions that can provide 14 tomographic slices. The spatial resolution is 6.7 mm full width at half maximum. The sensitivity is 125 kilocounts/second per microcuries/milliliter (200 mm water). Studies were performed after fasting for at least 13 h. If the serum glucose level < 120 mg/mL, 50–75 g glucose were ingested, and sequential measurements of serum glucose were made. If the serum glucose level > 160 mg/mL, serum glucose was adjusted by injection of insulin. When the serum glucose level reached 120–160 mg/mL, 296–370 MBq (8–10 mCi) FDG (Chinese Atomic Energy Institute; Beijing, China) were given intravenously. The serum glucose level (mean ± SD) was 136 ± 30 mg/mL. One hour after FDG administration, data were acquired with a 128 × 128 matrix (140 mm) over 20–25 min (approximately 20 million time coincidence counts). Transaxial images were reconstructed using the Butterworth-filtered backprojection method (order, 5; cutoff frequency, 0.3). Horizontal long-axis, vertical long-axis, and short-axis images were obtained. The thickness of each tomographic slice was 3.33 mm, and 2 PET slices were combined to achieve a thickness comparable with that of SPECT (6.7 and 6.4 mm, respectively) (6,7).
99mTc-MIBI SPECT Perfusion Imaging
Two hours after intravenous injection of 740–925 MBq (20–25 mCi) 99mTc-MIBI (Radiation Chemistry Department, Beijing Normal University, Beijing, China) at rest, myocardial perfusion imaging was performed using a multi-SPECT 3 scanner (Siemens Medical Systems, Hoffman Estates, IL) equipped with a low-energy, all-purpose collimator centered on the 140-keV photopeak with a 20% symmetric energy window. Sixty images covering 360° were acquired with a 64 × 64 matrix. The transaxial data were reconstructed using the Butterworth-filtered backprojection method with a cutoff frequency of 0.45 and an order of 7. Tomographic images were displayed as short-axis, horizontal, and vertical long-axis slices.
Image Analysis
The images of left ventricle obtained from 99mTc-MIBI SPECT and FDG PET were divided into 9 segments (apex, anterior, anterobasal, inferior, posterior, anteroseptal, inferoseptal, anterolateral, and inferolateral). Perfusion and metabolism images were evaluated by 2 experienced nuclear medicine physicians who were unaware of the clinical data. The myocardial uptakes of 99mTc-MIBI and FDG were assessed using a 4-point scoring system (0 = normal tracer uptake, 1 = moderate reduction in tracer uptake, 2 = severe reduction in tracer uptake, and 3 = absence of tracer uptake). If ≥2 continuous slices had a score >1, the image was defined as abnormal and a single segment with a score of 2 or 3 was also defined as abnormal.
In normal myocardial images, uptake of 99mTc-MIBI or FDG was relatively uniform, but in abnormal radioactive distribution, 2 different patterns of 99mTc-MIBI and FDG uptake were observed. Regions with perfusion defect but preserved FDG uptake were defined as perfusion–metabolism mismatch (MM), indicating jeopardized but viable myocardium. Regions with perfusion defect and matched decrease of FDG uptake were defined as match (M), indicating myocardial necrosis (Figs. 1 and 2).
99mTc-MIBI SPECT and FDG PET images of patient with MI. (A) Perfusion–metabolism MM at apex, anterior, septal, and inferoposterior walls (arrows) before revascularization. (B) After CABG, perfusion defects of segments improved significantly and size of left ventricle decreased. EF increased from 30% to 42%. ST = stress; RE = rest.
Echocardiography
Resting 2-dimensional echocardiography was performed with a Toshiba-380 system (Toshiba, Tokyo, Japan). The short axes of the left ventricle were obtained from the parasternal region at 3 levels: the mitral valve, high muscle, and midpapillary muscle. The long axes were obtained at the apical 4-chamber and 2-chamber views. EF was calculated using Sympson’s method. In addition, the LV end-diastolic diameter (EDD) was measured. Regional wall motion of 9 segments (as for MIBI SPECT) was assessed using a 4-point scoring system (1 = normal, 2 = hypokinetic, 3 = akinetic, and 4 = dyskinetic).
Follow-Up
The echocardiography studies were repeated at 3 mo (Pos1) and 6 mo (Pos2) after revascularization.
Follow-up data were obtained by review of patients’ clinical records and phone contact with the patients or their relatives. The average follow-up period was 26 ± 10 mo (range, 1–36 mo; median, 28 mo). Cardiac death, acute MI, unstable angina requiring revascularization, and late revascularization (>3 mo after radionuclide imaging) experienced by the patients were considered as cardiac events.
Statistical Analysis
Data analysis was performed using SPSS version 9.0 (SPSS Inc., Chicago, IL) software. All data are expressed as mean ± SD, with categoric data expressed as a percentage. Comparisons between groups with continuous data were made by the unpaired Student t test. A χ2 test was used for categoric variables (with Yates’ correction or Fisher’s exact test in smaller sizes). P < 0.05 was considered statistically significant.
Cox proportional hazards regression analysis was applied to determine the independent predictors of cardiac events. Multivariate data analysis was performed in a forward-stepwise fashion. The clinical variables included age, gender, risk factors (such as smoking, family history of CAD, hypertension, diabetes mellitus, and hyperlipidemia), prior MI, LV aneurysm, New York Heart Association (NYHA) heart failure class, and Canadian Cardiovascular Society (CCS) angina class. The LV functional variable was EF. The imaging variable was the number of MM segments. The coronary angiography variable was the number of diseased vessels and the final variable was whether the patient underwent revascularization.
Event-free survival curves and survival curves were constructed using the Kaplan–Meier method and were compared with the log rank test.
RESULTS
Clinical Characteristics
The final study group comprised 123 patients (114 men, 9 women; mean age, 56 ± 9 y) with a mean EF of 35% ± 6%. Fifty-two patients had a history of hypertension (42.3%), 56 had a history of smoking (45.5%), 16 had diabetes (13.0%), 26 had hyperlipidemia (21.1%), and 25 had family history of CAD (20.3%). Ninety-five patients had Q-wave MI (77.2%) and 41 had LV aneurysm (33.3%). Sixty-four patients had NYHA heart failure class III–IV (52.0%) and 106 had CCS angina class II–IV (86.2%). Sixty-eight patients had 3-vessel disease (55.3%), 7 had left main CAD (5.7%), 41 had 2-vessel disease (33.3%), and 14 had 1-vessel disease (11.4%).
Sixty-seven patients underwent revascularization (54.5%) either by PTCA (n = 9) or by CABG (n = 58). Fifty-six patients received medical therapy (45.5%). Patients with >2 MM segments indicating myocardial viability were classified as group A, and patients with <2 MM segments indicating myocardial necrosis were classified as group B. According to the treatment strategy, patients who underwent revascularization were assigned as group A1 (n = 42) and group B1 (n = 25) and those treated medically were assigned as group A2 (n = 30) and group B2 (n = 26). The clinical and angiographic characteristics are shown in Table 1. No significant differences were found between groups A1, A2, B1, and B2 in terms of age, gender, risk factors, NYHA heart failure class, CCS angina class, EF, EDD, and number of vessels involved.
Clinical and Angiographic Data
Changes of LV Function Before and After Revascularization
After revascularization, EF of group A1 increased from 36% ± 5% to 44% ± 8% (P < 0.0001) in Pos1 and to 51% ± 9% (P < 0.0001) in Pos2. In addition, EF in Pos2 was higher than that in Pos1 (P = 0.02). EDD decreased from 62 ± 8 mm to 56 ± 5 mm (P < 0.001) in Pos1 and to 55 ± 5 mm (P < 0.001) in Pos2. However, the EF and EDD of group B1 had no significant changes after revascularization (P > 0.05) (Fig. 3).
99mTc-MIBI SPECT (A) and FDG PET (B) images of patient with MI show perfusion–metabolism M in inferoposterior wall (arrows) before revascularization. (C) After CABG, perfusion defects did not improve and EF did not change (38% vs. 39%).
Changes of EF (%) and EDD (mm) at baseline (Pre), 3 mo (Pos1), and 6 mo (Pos2) after revascularization in groups A1 and B1.
Prediction of Cardiac Events
During follow-up, 22 patients suffered from cardiac events (17.9%), including 11 cardiac deaths (50%), 4 acute MI (18.2%), 6 late CABG (27.3%), and 1 unstable angina (4.5%). The cardiac event rate of patients who received medical therapy was significantly higher than that of patients who underwent revascularization (32.1% vs. 6%; χ2 = 14.23; P = 0.001).
The cardiac event rate of patients with viable myocardium and who received medical therapy (group A2) was significantly higher than that of groups A1 (50% vs. 2.4%; χ2 = 23.08; P < 0.0001), B1 (50% vs. 12%; χ2 = 8.94; P = 0.003), and B2 (50% vs. 11.5%; χ2 = 9.45; P = 0.002). However, no statistical differences were found between groups of A1, B1, and B2.
The cardiac mortality rate of group A2 was significantly higher than that of groups A1 (26.7% vs. 0%; χ2 > 48.8; P < 0.0000) and B2 (26.7% vs. 4%; χ2 = 5.38; P = 0.02).
Univariate analysis showed that the CCS angina class (P < 0.05) and number of MM segments (P < 0.05) were predictive of cardiac events, and the NYHA heart failure class (P < 0.005) and EF (P < 0.01) were predictive of cardiac mortality. However, revascularization had a positive effect on cardiac event-free survival (χ2 = 13.3; relative risk [RR] = 0.17; 95% confidence interval, 0.06–0.52; P < 0.001) and on survival (χ2 = 6.56; RR = 0.18; 95% confidence interval, 0.04–0.82; P < 0.05).
Multivariate analysis showed that the number of MM segments, CCS angina class, and NYHA heart failure class were independent predictors for cardiac events. The NYHA heart failure class was an independent predictor for cardiac mortality (P < 0.001) (Table 2).
Independent Predictors of Cardiac Event and Cardiac Mortality by Cox Multivariate Analysis
Estimated Event-Free Survival Curves and Survival Curves
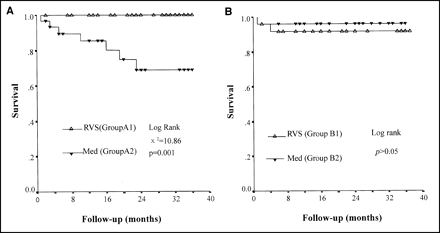
The estimated event-free survival rates of group A2 were 66%, 48%, and 48% at 1, 2, and 3 y of follow-up, respectively. However, estimated event-free survival rates of group A1 were 100%, 100%, and 92% during the same periods (P < 0.0001). No significant difference of event-free survival rates was found between groups B1 and B2 (Fig. 4).
Cumulative cardiac event-free survival curves of groups A1 and A2 (A) and groups B1 and B2 (B). RVS = revascularization; Med = medical therapy.
The estimated survival rates of group A2 were 85%, 69%, and 69% at 1, 2, and 3 y of follow-up, respectively. In contrast, the estimated event-free survival rates of group A1 were 100%, 100%, and 100% during the same periods (P = 0.001). No significant difference of survival rates was found between groups B1 and B2 (Fig. 5).
Cumulative survival curves of groups A1 and A2 (A) and groups B1 and B2 (B). RVS = revascularization; Med = medical therapy.
Cardiac Events in Patients With and Without Aneurysm
The cardiac event rates and cardiac mortality of patients with LV aneurysm (n = 41) and without aneurysm (n = 82) were 24.4% versus 14.6% (P > 0.05) and 12.1% versus 7.3% (P > 0.05), respectively.
The estimated cardiac event-free survival rate of patients with aneurysm and viable myocardium who underwent revascularization (n = 8) was significantly higher than that of patients who received medical therapy (n = 13) (100% vs. 46%; χ2 = 7.06; P < 0.005). Patients with aneurysm and no viable myocardium who underwent revascularization (n = 10) did not show a decrease in the cardiac event-free survival rate compared with those who received medical therapy (n = 10) (80% vs. 80%; P > 0.05) (Fig. 6).
Cumulative cardiac event-free survival curves of patients with aneurysm and viable myocardium (A) and patients with aneurysm and no viable myocardium (B). RVS = revascularization; Med = medical therapy.
DISCUSSION
Patients with previous MI and LV dysfunction usually have poor prognoses when treated medically (15). Coronary revascularization could improve the survival rate and LV function (16,17). However, patients suffered high risks from revascularization, especially those with severe LV dysfunction (18). Therefore, evaluation and management of patients with MI and LV dysfunction are very important issues for decreasing the cardiac mortality and improving the quality of life (2,3). Several studies have shown that the use of 99mTc-MIBI SPECT and FDG PET is comparable with 13N-ammonia and FDG PET for assessment of myocardial viability in patients with CAD and LV dysfunction and identification of patients who are suitable for revascularization (4–9). However, the role of the hybrid technique of 99mTc-MIBI SPECT and FDG PET in evaluation of the prognosis, treatment strategy, and improvement of LV function of patients with MI and LV dysfunction is still uncertain. Our study showed that the presence of myocardial viability (≥2 MM segments) in patients with MI and LV dysfunction who underwent medical treatment (group A2) usually was associated with poor prognoses, and such patients could benefit most from revascularization (group A1).
Using the Cox proportional hazards model, our study showed that the number of MM segments, CCS angina class, and NYHA heart failure class had negative effects on the cardiac events (P < 0.05), and the NYHA heart failure class and EF had negative effects on the cardiac mortality (P < 0.01), whereas revascularization had a positive effect on the clinical outcome (P < 0.01). Patients who underwent revascularization had a higher event-free survival rate compared with those who were treated medically (94% vs. 68%; P = 0.001). The results are consistent with the findings of a previous study (10).
According to the 99mTc-MIBI SPECT and FDG PET results and treatment strategy, patients with MI and LV dysfunction had different event-free survival rates and survival rates. Kaplan–Meier analysis showed that patients with myocardial viability who underwent revascularization (group A1) had a higher estimated 3-y event-free survival rate (92%) and a higher survival rate (100%). In contrast, patients with myocardial viability who received medical therapy (group A2) had the lowest estimated 3-y event-free survival rate (48%) and a low survival rate (69%) (P < 0.01). However, patients without myocardial viability had similar 3-y event-free survival rates (88% vs. 88.5%) and similar survival rates (92% vs. 96%) whether patients underwent revascularization or received medical therapy (Figs. 4 and 5). Therefore, patients with myocardial viability, assessed by 99mTc-MIBI SPECT and FDG PET, have the greatest benefit from coronary revascularization.
These findings are concordant with the results of previous studies, which showed that patients with viable myocardium were at high risk for future cardiac events if they did not undergo revascularization (11,19–22). Di Carli et al. (21) have shown that patients with an MM pattern who underwent revascularization had a higher survival rate than those who were treated medically (88% vs. 50%). Recently, the same group reported that the 4-y survival rate of patients with viable myocardium who received bypass grafting was much higher than that of patients who received medical therapy (75% vs. 30%) (22). vom Dahl et al. (23) showed that the rate of cardiac events in patients with viable myocardium who were treated medically was 22%; however, those patients benefited from coronary revascularization with a cardiac event rate of 0%. In addition, the cardiac mortality of patients with viable myocardium who underwent early revascularization (<35 d after PET study) was lower than that of patients who underwent delayed revascularization (≥35 d after PET study) (0% vs. 24%; P < 0.05) (24). The earlier revascularization was performed, the more improvement of LV function would be achieved (24,25). These studies differed from ours in that 13N-ammonia or rubidium rather than 99mTc-MIBI was used as the perfusion agent (11,20–22). 13N-ammonia or rubidium has a very short half-life and requires a cyclotron in the hospital for production. The cost of 13N or 82Rb is much higher than that of 99mTc-MIBI, which is the most cost-effective radiopharmaceutical for this purpose.
In addition, we assessed the relationship between the changes of LV function after revascularization and the extent of viable myocardium. Our study showed that patients with >2 viable myocardium segments had significant improvement of LV function after revascularization. Three months after revascularization, EF increased significantly (from 36% ± 5% to 44% ± 8%; P < 0.0001), and EDD decreased significantly (from 62 ± 8 mm to 56 ± 5 mm; P < 0.001). In addition, EF increased further to 51% ± 9% at 6 mo (vs. Pos1; P = 0.02), indicating that improvement of LV function was a sustained process after revascularization. However, the LV function of patients with necrotic myocardium was not improved after revascularization (P > 0.05) (Fig. 3).
Previous studies also revealed that the improvement of LV function was related to the amount of viable myocardium. A study by Cuocolo et al. (15) showed that the extent of viable myocardium was the best predictor of EF improvement and the strongest predictor for survival in patients with MI. Tillish et al. (26) showed that EF improved from 30% ± 11% to 45% ± 14% after bypass surgery only in patients with >2 MM segments. Recently, Di Carli et al. (21) reported that the improvement of heart failure symptoms was correlated with the intensity and severity of myocardial viability. However, this study did not assess the changes of LV function.
The limitation of our study is that patients with an LV aneurysm were included. It is well known that patients with an LV aneurysm have LV remodeling and poor ventricular function that would make our study complex and might have some impact on the results. However, the presence of the aneurysm did not make a difference in terms of outcome. Statistical analysis showed that the cardiac event rate and cardiac mortality were not significantly different in patients with and without an LV aneurysm. However, the cardiac event rate of patients with viable myocardium and an aneurysm who underwent revascularization was much lower than that of patients who were treated medically (0% vs. 54%; P < 0.005). In contrast, the prognosis of patients with an aneurysm and no viable myocardium was not improved even if revascularization was performed. Therefore, the prognosis of patients with an aneurysm correlated with the extent of viable myocardium and treatment strategy.
CONCLUSION
Assessment of myocardial viability using hybrid 99mTc-MIBI SPECT and FDG PET can predict the clinical outcome and is helpful to decision making in the treatment strategy for patients with MI and LV dysfunction. Revascularization can improve the LV function and clinical outcome of patients with >2 viable myocardial segments.
Acknowledgments
The authors thank Yunjian Zhao, PhD, and his colleagues for excellent technical assistance.
Footnotes
Received Nov. 14, 2000; revision accepted Apr. 9, 2001.
For correspondence or reprints contact: Xiu-Jie Liu, MD, Department of Nuclear Medicine, Cardiovascular Institute and Fu Wai Hospital, Chinese Academy of Medical Sciences, Peking Union Medical College, Beijing, 100037, China.