Abstract
99mTc-RP128 is a bifunctional peptide chelate designed to target the tuftsin receptor, expressed by neutrophils, monocytes, and macrophages. Studies in animal models of both infectious and noninfectious inflammation have shown a positive correlation between accumulation of 99mTc-RP128 and quantitative measures of inflammation. A phase 1 trial was conducted with the objective of determining the safety, biodistribution, and human dosimetry of 99mTc-RP128 in eight healthy volunteers. For evaluation of the potential of 99mTc-RP128 for imaging sites of inflammation, 10 patients with active rheumatoid arthritis were studied. Methods: Normal biodistribution was determined using the conjugate view method up to 24 h after intravenous injection of 280 MBq 99mTc-RP128. Dosimetry calculations were based on standard MIRD methodology, using the International Commission on Radiological Protection model 30 of the gastrointestinal tract and a voiding bladder model with an interval of 4.8 h. For rheumatoid arthritis patients, whole-body scans and spot views of the hands, knees, and feet were obtained at 1 and 3 h after injection of 475 MBq 99mTc-RP128. Results: 99mTc-RP128 was cleared rapidly from the blood by renal excretion, and no major organs showed significant accumulation. The synovia of the major joints were visualized for all subjects. The effective dose equivalent and the effective dose were calculated to be 0.011 and 0.0094 mSv/MBq, respectively. The highest dose was to the bladder wall, which received 0.076 mGy/MBq. In all rheumatoid arthritis patients, we observed a markedly increased uptake in several affected joints. Painful and swollen joints were detected with a sensitivity of 76% and 69%, respectively. Seventy-three percent of the joints with radiographic signs of erosion were scintigraphically positive. In some patients, lines of increased activity were observed and were considered to correspond to uptake in the synovium lining tendon sheaths in the wrists and hands. Conclusion: This study shows that 99mTc-RP128 is safe and can successfully be used to visualize clinically affected joints in patients with long-standing rheumatoid arthritis. A proposed radioactive dose of 450–500 MBq will produce an effective dose well within the range of effective doses for commonly used radiopharmaceuticals.
The most commonly used agents in the scintigraphic diagnosis and localization of inflammatory conditions are 111In- or 99mTc-labeled white blood cells and 67Ga-citrate. However, although these agents are widely accepted, none is considered satisfactory. The preparation of radiolabeled white blood cells involves isolating cells from the patient’s blood—a time-consuming process that also runs the risk of infecting the staff and patients with blood-borne pathogens. Images obtained with 67Ga-citrate are relatively nonspecific, and the imaging time is lengthy. 99mTc-labeled antigranulocyte antibodies allow in vivo labeling of white blood cells (1,2). However, these agents, although relatively simple to prepare, generally have slow clearance times that delay imaging. In addition, they may show an uptake pattern related to the physiologic distribution of leukocytes (in spleen and bone marrow), limiting their use to particular anatomic locations. Thus, a need exists for imaging agents that are easy to prepare and have improved specificity and shortened imaging times. The use of radiolabeled receptor-targeted peptides can potentially fulfil these requirements.
RP128 is a small, bifunctional peptide chelate designed as a tuftsin receptor antagonist to provide rapid imaging of inflammation. Tuftsin is a leukokinin-derived tetrapeptide that promotes phagocytosis and chemotaxis of neutrophils, monocytes, and macrophages by a receptor-mediated mechanism (3). The tuftsin receptor was chosen as the target because it is located exclusively on these phagocytic cells, which accumulate at sites of inflammation. RP128 (Fig. 1) consists of a tripeptide N3S 99mTc-chelator, dimethyl-Gly-Ser-Cys (acetamidomethyl), linked by a glycine residue to the targeting domain composed of the tuftsin antagonist Thr-Lys-Pro-Pro-Arg (TKPPR). Chelation with 99mTc results in loss of the protecting acetamidomethyl group from the chelator sequence. The pentapeptide analog TKPPR has a fourfold greater receptor affinity than does tuftsin (4). In vitro binding studies showed that the metal (ReO) complex of RP128 binds to the tuftsin receptor with the same affinity as TKPPR (Resolution Pharmaceuticals Inc., unpublished data, 1995). Preclinical testing in animal models of both infectious and noninfectious inflammation have shown a positive correlation between accumulation of 99mTc-RP128 and quantitative measures of inflammation. Because of the rapid renal excretion and low accumulation in other major organs, 99mTc-RP128 provided images of inflammation rapidly and with high target-to-background ratios that could be maintained for 18 h (Resolution Pharmaceuticals Inc., unpublished data, 1995). No signs of toxicity were observed when RP128 was administered as a single dose of 30 mg/kg or as daily doses of 3 mg/kg for 14 consecutive days to rats and dogs. These dosages are, respectively, 1,000 times and 100 times the maximum anticipated human dose.
Chemical structure of 99mTc-RP128 complex.
The primary objective of this study was to investigate the safety, tolerance, normal biodistribution, and dosimetry of 99mTc-RP128 in healthy volunteers. To determine whether favorable inflammation imaging characteristics could be obtained in humans, we also evaluated the potential of 99mTc-RP128 for visualizing the inflamed joints of patients with active rheumatoid arthritis.
MATERIALS AND METHODS
Radiopharmaceutical Preparation
Lyophilized kits containing 2.0 mg RP128 peptide chelate, 30.0 mg sodium gluconate, and 0.2 mg stannous chloride were supplied by Resolution Pharmaceuticals Inc. Three to 4 GBq (80–100 mCi) 99mTcO4− in 4 mL isotonic saline, obtained from a commercial 99Mo–99mTc generator, were added to the vial under aseptic conditions, gently mixed with the reaction mixture, and incubated for at least 30 min at room temperature. The reconstituted solution was used within 6 h of preparation. A three-strip chromatographic system was used to assess the radiochemical purity of the 99mTc-RP128 preparation. Small samples of the reconstituted kit were spotted on the respective strips; the strips were cut after elution as appropriate and counted in a gamma well counter. An instant thin-layer chromatography silica gel strip (Pall Gelman Laboratory, Ann Arbor, MI) was developed in 0.9% sodium chloride. 99mTc-pertechnetate and 99mTc-gluconate migrate to the solvent front (A). Paper chromatography (Whatman, Ann Arbor, MI) was performed with 0.9% saline:0.1% trifluoroacetic acid as the mobile phase. In this system, reduced hydrolyzed 99mTc remains at the origin (B). The percentage of 99mTc-pertechnetate was measured by eluting an instant thin-layer chromatography SG strip in acetone, for which 99mTc-pertechnetate moves to the solvent front. The radiochemical purity, or percentage of 99mTc-RP128, was then calculated as 100% − B − A. The radiochemical purity was higher than 90% for all radiolabeled kits.
Biodistribution Study
Study Protocol.
Eight healthy male volunteers (age range, 20–30 y; mean age, 24 y) participated in the biodistribution study. No subjects had a history of disease or a known allergy to peptides, nor were any taking medication before entering the study. The use of concomitant medication was strictly prohibited throughout the study. Written informed consent was obtained from each volunteer, and the protocol was reviewed and accepted by the institutional review board of the university.
Ten minutes before the study, an 18-gauge intracatheter line was installed in an antecubital vein for administration of the tracer, and a similar venous access was installed in a contralateral vein for blood sampling. Blood samples were taken for biochemical analysis 10 min before administering the material and again 24 h after injection. Vital signs, including blood pressure, pulse, respiration, and temperature, were recorded 15 min before injection and again 15–40 min after tracer administration. 99mTc-RP128 was administered as a single intravenous injection followed by 10 mL saline. The injected activity ranged from 259 to 296 MBq (mean ± SD, 282 ± 12.7 MBq). The net weight of peptide administered was either 150 μg (five subjects) or 200 μg (three subjects). Blood samples were collected from the contralateral arm directly before injection and at 2, 5, 10, 20, 30, and 60 min and 2, 4, 8 and 24 h after injection. Urine samples were collected by the subjects ad libitum for 24 h after injection. Three subjects collected all feces until 24 h after injection.
Whole blood and plasma samples as well as aliquots of the urine samples were counted against appropriate standards of known dilution in an automatic gamma well counter and, after correction for decay and background activity, expressed as a percentage of the injected dose. The blood volume of each volunteer was estimated according to body weight and height to determine the individual-volunteer blood dilution factor for blood clearance analysis. Fecal specimens were counted in duplicate on the gamma camera for 2 min each with an appropriate standard.
Imaging.
Anterior and posterior whole-body scans were obtained with a dual-head Bodyscan camera (Siemens Inc., Hoffman Estates, IL) equipped with parallel-hole low-energy collimators and connected to a data processing system using the commercial Icon software (Siemens Inc.). These whole-body measurements were made at 10, 30, and 60 min (10-min imaging) and at 3, 6, 8, and 22–24 h (20-min imaging) after injection.
Regions of interest were drawn on the 10-min anterior and posterior views, and the total number of counts over the whole body was considered 100% of the injected dose. The same regions were used on all subsequent images. For attenuation correction, the geometric mean of the anterior and posterior counts was calculated, and the fraction of activity remaining in a particular organ was then measured by comparing the geometric mean counts in the region of interest with the estimate of total body counts. Specific regions of interest were drawn for the brain, thyroid, lungs, heart, liver, spleen, kidneys, urinary bladder, gonads, and knee joints. For the estimation of intestinal activity, a region of interest including the whole abdomen was drawn. The total number of counts in this region minus the number of counts in the liver, spleen, and kidneys was considered to represent intestinal activity.
Dosimetry Calculations.
Radiation dose estimates for the adult male human were calculated from the biodistribution data using standard methodology set forth by the MIRD committee and the International Commission on Radiological Protection (ICRP) (Appendix). The ICRP model 30 of the gastrointestinal tract and a voiding bladder model with voiding intervals of 4.8 h were used.
Patient Studies
Study Protocol.
Ten rheumatoid arthritis patients (two men, eight women) entered and completed the study. Written informed consent was obtained from each patient. All the patients fulfilled the 1987 American Rheumatism Association criteria. One inclusion criterion for the study was a positive rheumatoid factor (>22 IU/mL). Plasma rheumatoid factors for the 10 patients are summarized in Table 1. On the study day, all major joints were scored for pain and swelling by the same rheumatologist. Pain was scored using the articular index of Ritchie (5), in which 0 = no painful sensation; 1 = painful sensation; 2 = painful sensation and wince; and 3 = painful sensation, wince, and withdrawal. Swelling was scored on a 0–3 scale, with 0 representing no swelling and 3 representing intense swelling. These scores were also recorded as negative or positive for each joint, with a score of 0 taken as negative and a score of 1 or greater taken as positive. A radiographic evaluation of the patient’s hands and wrists was also performed on the day of the study, and the presence or absence of joint erosion was recorded for the major joints.
Plasma Rheumatoid Factor of 10 Rheumatoid Arthritis Patients
After the physical examination, an echocardiogram was obtained for each patient. Blood pressure, pulse, respiration, and temperature were recorded 15 min before injection and again later during the study day. Ten minutes before injection, an appropriate intracatheter line was inserted into an antecubital vein for administration of the tracer, and a similar venous access was installed in the contralateral vein for blood sampling. Blood and urine samples were taken for biochemical analysis before injection of the drug. For follow-up, all patients returned within 6 d after the injection, and blood and urine sampling was repeated for biochemical analysis.
An average dose of 475 ± 9.9 MBq (range, 452–486 MBq) 99mTc-RP128 was administered as a single intravenous bolus injection followed by 10 mL saline. Blood samples were taken for blood clearance measurements at 0.5, 1, and 3 h after tracer injection. Blood sample counting was performed as already described.
Imaging.
Anterior and posterior whole-body scans were obtained at 0.5, 1, and 3 h after injection (scanning time = 10 min). After the 1- and 3-h whole-body acquisitions, spot images of the hands, knees, and feet were acquired with an Orbiter camera (Siemens) or with a Sophy single-head camera (Sopha Medical Vision, Paris, France) equipped with a parallel-hole low-energy collimator.
The scintigrams of all patients were analyzed by the same observer, who was unaware of clinical findings. The joints were scored on a 0–3 scale, in which 0 = no abnormal uptake; 1 = low uptake, probably normal; 2 = moderate uptake, probably abnormal; and 3 = definitely abnormal uptake. The presence or absence of abnormal accumulation of tracer in each joint was calculated, with a score of 0 or 1 taken as negative and a score of 2 or 3 taken as positive. To compare the number of lesions visualized with those clinically and scintigraphically assessed, different groups of joints were considered. All subsequent calculations used scintigraphy data from the 1-h whole-body or spot scans, with the exception of one patient whose 1-h spot scans were not available. For this patient, data from the 3-h spot scans were used. The relationship with pain was evaluated for the shoulder, elbow, wrist, metacarpophalangeal (MCP) and proximal interphalangeal (PIP) joints (counted as one joint), knee, and ankle (total number of joints, 140). Swelling was individually assessed for the elbow, wrist, first to fifth MCPs, first to fifth PIPs, knee, and ankle (total number of joints, 280). Scintigraphy and erosion were compared for the wrist, first to fifth MCPs, and first to fifth PIPs (total number of joints, 220).
RESULTS
Safety Data
The injection of 99mTc-RP128 was well tolerated, and no adverse reactions or changes in vital signs related to the injection were observed in any of the volunteers or patients. No changes in blood and biochemical parameters were observed that could be related to the administration of the radiopharmaceutical. All laboratory values outside the normal range were related to the disease state of the patients. No adverse events were reported.
Normal Biodistribution and Dosimetry
Blood Clearance and Excretion.
The labeled molecule was cleared rapidly from the blood and followed a biphasic clearance curve (Fig. 2). Fitting the blood clearance data to a biexponential function, the half-lives of distribution and elimination of 99mTc-RP128 were calculated to be 4 min and 69 min, respectively. Blood activity was found mainly in the plasma, indicating almost no uptake in the cellular fraction of the blood. After 2–4 h, 64.3% ± 10.7% had been excreted into the urine. At 22–24 h, 1.0% ± 0.4% of the injected dose remained in the blood and 81.4% ± 10.9% had been excreted into the urine. Cumulated fecal activity at 22–24 h was 1.57% ± 0.2% (n = 3).
Blood and plasma clearance of 99mTc-RP128 in healthy volunteers.
Biodistribution.
The relative biodistribution expressed as the percentage of organ uptake versus time is given in Table 2. With the exception of the kidneys and urinary bladder, no significant accumulation occurred in any major organs. In all subjects, the synovia of the joints were visualized. The disappearance rate of the tracer in the brain, thyroid, lung, heart, liver, and spleen paralleled the plasma disappearance curve. Synovial activity washout from the knee joints was slower, allowing high target-to-background spot images of the knee synovial tissue 1 and 3 h after injection. 99mTc-RP128 was excreted mainly through the kidneys and the bladder. Anterior whole-body images at different times after tracer administration are shown in Figure 3.
Anterior whole-body images of healthy volunteer at 30 and 60 min and at 3 and 6 h after 99mTc-RP128 administration
Biodistribution of 99mTc-RP128 in Healthy Volunteers at Different Times After Intravenous Injection
Dosimetry.
The absorbed dose estimates for different organs are summarized in Table 3. The effective dose equivalent and effective dose were calculated to be 0.011 and 0.0094 mSv/MBq, respectively. The highest dose was to the bladder wall, which received 0.076 mGy/MBq.
Radiation Dose Estimates for 99mTc-RP128 for Adult Male Human Based on MIRD Method
Rheumatoid Arthritis Patient Studies
Blood Clearance.
The initial blood clearance at 30 min after tracer administration was significantly slower and more variable (27.8% ± 14.5%) than in the healthy volunteers (17.7% ± 2.6%), but the remaining blood activity at 1 h was the same. No relationship was apparent between slower initial clearance and the number of affected joints. The differences in clearance may have been related to much larger differences in renal function in the rheumatoid arthritis patients than in the younger healthy volunteers.
Scintigraphic Evaluation.
In all patients with rheumatoid arthritis, we observed a markedly increased uptake in several affected joints compared with the normal moderate synovial uptake observed in healthy volunteers. The distribution of this increased joint activity varied from patient to patient and from one affected joint to another within the same patient. In some patients, lines of increased activity were observed between joints and were considered to correspond to uptake in the synovium lining tendon sheaths in the wrists and hands (Fig. 4). In one patient, for whom a recent bone scintigram was available, a marked discrepancy was seen between the intense, symmetric involvement of nearly all joints on the bone scan and the distribution of the pathologic foci on 99mTc-RP128 scintigraphy.
Spot image of hands and wrists of patient with rheumatoid arthritis. Lines of increased activity between joints may correspond to uptake in synovium lining tendon sheaths.
The results of joint examination and joint 99mTc-RP128 scintigraphy of the 10 patients with rheumatoid arthritis (20 joint groups) are summarized in Table 4. In this evaluation, the five MCP and five PIP joints were considered as one joint; the composite joint was scored positive if any one of the individual joints was scored positive. In this small study, we could not show a direct correlation between any of the clinical scores for pain, swelling, or erosion from patient to patient or from one affected joint to another within the same patient. Of the 100 joints that were scored for both pain and swelling (elbow, wrist, knee, MCP, and PIP, each scored as one joint), 47% had both pain and swelling, and 13% had neither pain nor swelling. Forty-seven of 64 painful joints (73%) were swollen, and 13 of 36 joints without pain (36%) were swollen. Forty-seven of 60 swollen joints (78%) were painful, and 17 of 40 joints without swelling (43%) were painful. Of the 220 joints scored for erosions, (wrist, first to fifth MCPs, and first to fifth PIPs), 104 (47%) were positive. Seventy-three percent of these eroded joints were positive for swelling, and 50% of the joints without erosions were swollen. Thirty-five percent of joints had both swelling and erosion. A comparison between pain and erosions was not meaningful, because pain was assessed for the MCPs and PIPs combined.
Relationship Between Number of Painful or Swollen Joints and Number of Scintigraphically Positive Joints on 99mTc-RP128 Images at 1 Hour After Injection
We could not show a direct correlation on a patient-by-patient basis between any of the total clinical scores of pain and swelling of the joints and the number of visualized joints with a score of 2 or 3. Table 5 illustrates the relationship between 99mTc-RP128 scintigraphy and the presence of pain, swelling, and erosion in individual joints. Sixty-six of 87 painful joints (76%) and 38 of 53 joints without pain (72%) were scintigraphically positive (score of 2 or 3). One hundred ten of 159 clinically swollen joints (69%) and 48 of 121 joints without swelling (40%) were scintigraphically positive. Seventy-seven of 106 joints (73%) with radiographic signs of erosion and 41 of 114 joints (36%) without signs of erosion were scintigraphically positive.
Relationship Between Positive 99mTc-RP128 Scintigraphy Findings and Joint Pain, Swelling, or Erosion
DISCUSSION
The purpose of the phase I study was to confirm the safety of 99mTc-RP128, to define its normal pharmacokinetics and biodistribution, and to calculate dosimetry in humans. RP128 was easily labeled with 99mTc at room temperature, yielding a complex with high radiochemical purity (>90%). No adverse events were associated with injection, and no changes in vital signs or laboratory tests were seen after injection. The study also showed that 99mTc-RP128 is cleared rapidly through the renal system, with no significant accumulation except in the kidneys and urinary bladder and the synovia of some joints, particularly the knees. Dosimetry calculations showed low dose accumulations to the major organs. As expected, the highest dose estimate was to the urinary bladder wall. A radioactive dose of 450–500 MBq will produce an effective dose of 4.5–5 mSv, which is well within the range of effective doses for commonly used radiopharmaceuticals (risk category I) (6).
These normal pharmacokinetics and biodistribution data are promising when 99mTc-RP128 is compared with other currently used imaging agents. The rapid blood clearance and renal excretion should allow earlier imaging with low background activity. The insignificant hepatobiliary or intestinal excretion and the absence of accumulation in the liver and spleen indicate that 99mTc-RP128 is potentially useful in abdominal imaging. Promising results were obtained from preclinical studies on a rat model of inflammatory bowel disease (7). 99mTc-RP128 could image the inflamed colon within 30 min of administration with higher target-to-background ratios than are obtained from 111In-leukocytes, which are currently used to image inflammatory bowel disease. In a pilot phase II study, 99mTc-RP128 was shown to visualize sites of active inflammation in patients with active Crohn’s disease (8).
The favorable biodistribution characteristics and the interesting synovial uptake of 99mTc-RP128 in several joints in healthy volunteers led us to investigate the potential of this tracer for imaging inflamed joints in patients with rheumatoid arthritis. Rheumatoid arthritis is a systemic inflammatory disorder principally involving the synovial membranes of multiple joints. The disease is chronic and has exacerbations and remissions. As the disease progresses, a distinction must be made between the active inflammation of synovial tissues and the secondary destructive changes found in later stages. The lack of a gold standard to quantify the activity of rheumatoid arthritis leaves joint pain and swelling as the main clinical indicators of inflammatory activity. Bone scanning is sometimes performed on patients with rheumatoid arthritis, but nuclear medicine does not play a major role either in the initial diagnosis or in subsequent assessments of patients.
In this small study, 99mTc-RP128 rapidly revealed the clinically affected joints in 10 patients with long-standing rheumatoid arthritis. The sensitivity of 99mTc-RP128 imaging for joint swelling and pain—respectively, 69% and 76%—is of a magnitude similar to that described for somatostatin receptor scintigraphy (76%) (9); however, the sensitivity is lower than that described for 99mTc-IgG (87%–92%) in several studies (10–12). This was a preliminary study, and alterations in the imaging protocol or increased experience with image interpretation might increase the calculated values. The sensitivity of 99mTc-RP128 scintigraphy for the detection of joints with bone erosions was 73%. 99mTc-IgG accumulation is reported to correlate with joint swelling, and the authors have conjectured that swelling is a better marker for active inflammation than is pain. This study showed similar sensitivities for pain or swelling compared with pain and swelling combined. In the absence of an accurate gold standard, it is difficult to attribute any physical observation with an ongoing inflammatory process, and presumably there is a certain degree of reporting difference of clinical observations between physicians.
Because no synovial biopsies were performed, definite statements on the true accuracy of 99mTc-RP128 imaging for the detection of persisting subclinical synovitis are difficult to make. Taking into account the high percentage of joints with false-positive findings, and considering that most of the patients were clinically stable, one might conjecture that 99mTc-RP128 imaging would be a sensitive parameter. Several factors indicate that 99mTc-RP128 might be a more sensitive indicator of synovitis lesions than of secondary bone destruction: the distribution patterns of increased uptake in the larger joints, the visualization of tendon sheaths rather than of individual small joints of the wrists and hands in some patients, and the discordance between bone scanning and 99mTc-RP128 scintigraphy findings in one patient. This possibility needs to be confirmed by histology.
Further evaluation of 99mTc-RP128 in rheumatoid arthritis will require a better understanding of the uptake mechanism in synovial tissue. Pharmacokinetic measurements of blood and plasma do not suggest a high degree of binding to circulating phagocytic cells. The increased uptake observed may, however, reflect different pathophysiologic mechanisms. In healthy volunteers, this uptake may be related to the normal presence of activated macrophages in these tissues. Alternatively, the physicochemical properties of the peptide chelate may favor accumulation in the protein matrix of the synovia. The number of activated macrophages is markedly increased in the rheumatoid synovial lining and subsynovial tissue, and macrophages have a central role in the development of bone erosions. In addition, neutrophils are frequently present in the synovial fluid of rheumatoid joints, reflecting ongoing active inflammation. 99mTc-RP128 has been specifically designed to target these cell lineages, and the tuftsin receptor has been reported to be upregulated in activated macrophages (13). The results of our study certainly do not contradict a preferential accumulation in those sites, although the mechanism of accumulation has yet to be explained.
CONCLUSION
99mTc-RP128 is a small peptide showing favorable biodistribution characteristics in humans. It is rapidly eliminated by renal excretion and does not significantly accumulate in the major organs. A proposed patient dose of 450–500 MBq will produce an effective dose well within the range of effective doses for commonly used radiopharmaceuticals. 99Tc-RP128 may be used as an effective inflammation imaging agent, although the mechanisms of uptake need further clarification.
APPENDIX
Dosimetry Calculations
Radiation dose estimates for 99mTc-RP128 were calculated by the Radiation Internal Dose Information Center on the basis of biodistribution data from eight male volunteers from the current study. From time–activity curves, residence times were determined for individual organs, the remainder of the body, and the excretory organs and were integrated in the MIRDOSE3.1 software, developed by the MIRD committee (14). The mean absorbed radiation dose (Dk) to a target organ (rk) is given by the following equation:
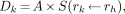 where A = cumulated activity in the source organ (rh) (MBq × h), defined by the administered activity and residence time in the source organ, and S = specific absorbed fraction to the target organ per unit cumulated dose in the source organ (mGy/MBq × h).
where A = cumulated activity in the source organ (rh) (MBq × h), defined by the administered activity and residence time in the source organ, and S = specific absorbed fraction to the target organ per unit cumulated dose in the source organ (mGy/MBq × h).
The residence time in the urinary bladder was calculated using a dynamic bladder model assuming a voiding interval of 4.8 h. Residence times for the gastrointestinal tract organs were determined with the ICRP model 30 of the gastrointestinal tract (15). This is a four-compartment model assuming transport of activity through the stomach, small intestine, upper large intestine, and lower large intestine and excretion in the feces. The model also allows for absorption from the small intestine into the blood. The effective dose and effective dose equivalent, which were calculated using weighting factors for the various organs, represent the overall internal dose as a single value and thus allow a comparison of the radiation risk from different diagnostic agents or different techniques involving ionizing radiation (e.g., nuclear medicine and radiography).
ACKNOWLEDGMENT
The authors thank the Radiation Internal Dose Information Center at the Oak Ridge Institute for Science and Education, Oak Ridge, TN, for dosimetry calculations.
Footnotes
Received Mar. 22, 2000; revision accepted Jun. 20, 2000.
For correspondence or reprints contact: Vicky Caveliers, RPh, Division of Nuclear Medicine, Free University Hospital Brussels (AZ-VUB), 101 Laarbeeklaan, B-1090 Brussels, Belgium.










