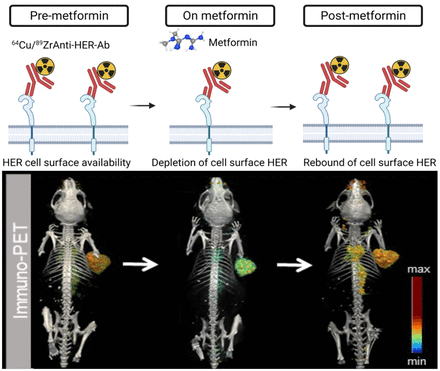
Visual Abstract
Abstract
Metformin has effects beyond its antihyperglycemic properties, including altering the localization of membrane receptors in cancer cells. Metformin decreases human epidermal growth factor receptor (HER) membrane density. Depletion of cell-surface HER decreases antibody–tumor binding for imaging and therapeutic approaches. Here, we used HER-targeted PET to annotate antibody–tumor binding in mice treated with metformin. Methods: Small-animal PET annotated antibody binding in HER-expressing xenografts on administration of an acute versus a daily dose schedule of metformin. Analyses at the protein level in the total, membrane, and internalized cell extracts were performed to determine receptor endocytosis, HER surface and internalized protein levels, and HER phosphorylation. Results: At 24 h after injection of radiolabeled anti-HER antibodies, control tumors had higher antibody accumulation than tumors treated with an acute dose of metformin. These differences were temporal, and by 72 h, tumor uptake in acute cohorts was similar to uptake in control. Additional PET imaging revealed a sustained decrease in tumor uptake on daily metformin treatment compared with control and acute metformin cohorts. The effects of metformin on membrane HER were reversible, and after its removal, antibody–tumor binding was restored. The time- and dose-dependent effects of metformin-induced HER depletion observed preclinically were validated with immunofluorescence, fractionation, and protein analysis cell assays. Conclusion: The findings that metformin decreases cell-surface HER receptors and reduces antibody–tumor binding may have significant implications for the use of antibodies targeting these receptors in cancer treatment and molecular imaging.
Metformin is the most prescribed first-line drug to reduce blood glucose levels in patients with type 2 diabetes mellitus (1,2). Metformin is safe, well tolerated, and prescribed to more than 120 million people for the management of type 2 diabetes mellitus. Metformin has benefits beyond its antihyperglycemic properties that include reduction of body weight and fat content, antiaging effects, and anticancer effects in patients with diabetes who are prescribed metformin (2–8). Patients with metabolic dysregulation (metabolic syndrome, diabetes, or obesity) who take metformin have shown lower cancer risk and cancer-related mortalities (7,9,10). As clinical trials repurpose metformin in oncology (11), it is important to understand how alterations induced by this drug on cancer cells and the tumor microenvironment affect tumor response to therapies (12,13).
Epidermal growth factor receptor (EGFR) and human epidermal growth factor receptor 2 (HER2) are receptor tyrosine kinases overexpressed and dysregulated in cancer cells (14). This has led to the development of small-molecule drugs and monoclonal therapeutic antibodies targeting human epidermal growth factor receptor (HER). Examples of anti-HER therapeutic monoclonal antibodies are trastuzumab and panitumumab, which target the extracellular domains of HER2 and EGFR in cancer cells (15,16). Trastuzumab is used as first-line therapy, in combination with chemotherapy, for gastric cancer and is the standard-of-care treatment for women with metastatic and early-stage breast cancer (15,17). Panitumumab is approved to treat wild-type Kirsten rat sarcoma viral metastatic colorectal cancer (18). The efficacy of HER-targeted antibody drugs depends on the density and availability of HER at the membrane of cancer cells (19,20). Previous studies suggest that drugs interfering with the stability and membrane localization of HER receptors will affect tumor response to antibody therapies.
Previous preclinical studies in gastric, breast, and bladder cancer models have shown that cholesterol-depleting drugs increase HER cell-surface density, as visualized by HER-directed immuno-PET uptake (19–21). These previous studies have also shown improved therapeutic efficacy of antibody drugs combined with cholesterol-depleting drugs (19–21). Accumulating preclinical and clinical data indicate that metformin interferes with the cholesterol biosynthetic pathway and raft production, but the molecular mechanisms of how these processes occur remain unclear (8,22–26). Metformin alters the cholesterol content located at the cell membrane and intracellularly, and it affects the synthesis and stability of receptors that rely on GM1 ganglioside, lipid raft markers (such as HER) (8,22–26). How the timing, duration, and dose of metformin treatment affect HER membrane receptor turnover in cancer cells is still unclear.
Immuno-PET is a useful tool for monitoring the uptake and binding of antibodies to tumors in real time. In this study, it was used to visualize and quantify the uptake of anti-HER antibodies in mice treated with metformin.
MATERIALS AND METHODS
Radiolabeling
89Zr-labeled trastuzumab and panitumumab were prepared with a specific activity of 22 MBq/nmol and immunoreactivity above 95%. 64Cu-labeled trastuzumab was prepared with a specific activity of 32–34.04 MBq/nmol. Detailed protocols are described in the Supplemental Methods (supplemental materials are available at http://jnm.snmjournals.org).
Metformin Treatment
In vitro treatments, Western blot, and immunofluorescence analyses are described in the Supplemental Methods. An acute dose of metformin (250 mg/kg) was orally administered 12 h before and at the same time as the tail vein injection of 89Zr-labeled antibody. Metformin (200 mg/kg dose) was then intraperitoneally administered for 7–11 consecutive days before the tail vein injection of 89Zr-DFO-trastuzumab or 89Zr-DFO-panitumumab. Detailed protocols are described in the Supplemental Methods.
Small-Animal PET and Biodistribution Studies
PET imaging experiments were conducted on a nanoScan PET/CT scanner (Mediso). Images were reviewed using 3D Slicer software (version 5.0.3; https://www.slicer.org/). The mice were sacrificed, and organs were harvested, weighed, and assayed in the γ-counter for biodistribution studies. Radioactivity associated with each organ was expressed as percentage injected dose (%ID) per gram of the organ.
Statistical Analyses
Data were analyzed using RStudio (Posit Software; http://www.rstudio.com/). Data are expressed as mean ± SD. Groups were compared using the Student t test. In biodistribution and imaging studies, each cohort included 3–4 mice per time point.
RESULTS
Metformin Induces Temporal Depletion of Cell-Surface HER2 or EGFR
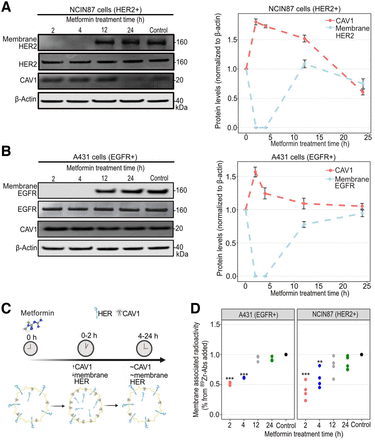
Trastuzumab or panitumumab binding to tumors depends on HER2 or EGFR availability at the membrane of cancer cells (19–21,27). Previous in vitro studies have shown alterations in membrane receptors in cancer cells treated with metformin in the millimolar range (28,29). We initially determined membrane levels of HER2 or EGFR in HER2-positive NCIN87 or EGFR-positive A431 cancer cells treated with 5 mM metformin (Fig. 1). We found that metformin promotes loss of cell-surface HER between 0 and 4 h of incubation time compared with no metformin (Figs. 1A and 1B; Supplemental Fig. 1). Metformin did not affect HER2 or EGFR in total protein lysates. The effect of metformin-induced membrane HER depletion was transient, and protein levels similar to those of control were detected after 12–24 h of incubation time.
(A and B) Western blot of total CAV1, membrane and total HER2, and membrane and total EGFR after cancer cell treatment with metformin. NCIN87 or A431 cancer cells were incubated with 5 mM metformin for 2, 4, 12, and 24 h. Graphs show quantification of Western blots with protein levels normalized to no-metformin control (bars, n = 3, mean ± SD). (C) Schematic representation showing metformin-induced temporal changes in CAV1 and membrane HER. Schematic was made using BioRender. (D) Fractionation assay for membrane-bound and internalized 89Zr-labeled trastuzumab or 89Zr-labeled panitumumab after incubation of cancer cells with 5 mM metformin for 2, 4, 12, and 24 h. **P < 0.01, based on Student t test and compared with untreated cells (n = 3–4, mean ± SD). ***P < 0.001, based on Student t test and compared with untreated cells (n = 3–4, mean ± SD).
Caveolin-1 (CAV1), a crucial structural protein of cholesterol-rich caveolae, negatively correlates with membrane HER at the protein level and affects anti-HER antibody binding to cancer cells (19,20,28,30–34). Previous studies have shown an upregulation in CAV1 tumoral levels on treatment with metformin. Because NCIN87 and A431 cancer cells are rich in caveolae (35), we next hypothesized that metformin-induced depletion in membrane HER is accompanied by an increase in total CAV1 protein levels. HER2-positive NCIN87 and EGFR-positive A431 cancer cells showed a 1.8-fold and 1.6-fold increase in CAV1 total protein levels at 2 h after metformin treatment (Figs. 1A and 1B). CAV1 total protein levels at 24 h after incubation with metformin were similar to those of no-metformin control.
We next evaluated whether metformin-induced depletion in membrane HER would hamper antibody binding to cancer cells (Fig. 1C). Cellular fractionation of NCIN87 or A431 cells incubated with 89Zr-labeled trastuzumab or panitumumab showed a significant decrease in membrane-bound radioactivity at 2 h after incubation with metformin (Fig. 1D). At 24 h after cell incubation with metformin, membrane-bound antibody resembled those found at no-metformin control cells, confirming the transience and temporality of the metformin cell treatments.
Altogether, these results suggest that metformin temporarily enhances CAV1 total protein levels and decreases membrane HER and anti-HER membrane-bound antibody to cancer cells.
Short-Term Cell Incubation with Metformin Enhances HER2 and EGFR Internalization
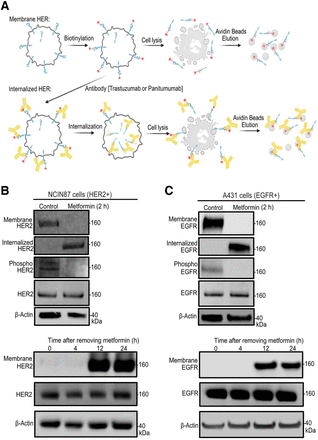
Premised on our initial in vitro findings, we anticipated that metformin-induced changes in membrane HER and total CAV1 protein levels would result in changes in HER internalization. This, in turn, would affect HER-mediated oncogenic signaling and the ability of trastuzumab or panitumumab to target and bind HER2- or EGFR-expressing cancer cells, respectively. Because we observed that short-term incubation with metformin decreased cell-surface HER and enhanced total CAV1 levels, we sought to investigate HER internalization and shedding in cancer cells treated with metformin for 2 h. Treatment with metformin did not induce significant changes in HER2 shedding in NCIN87 cancer cells (Supplemental Fig. 2). To determine HER intracellular localization, trastuzumab or panitumumab was allowed to bind on cancer cells treated with metformin for 2 h. Antibody-mediated internalization was then boosted in cancer cells for 90 min (Fig. 2A). Compared with untreated cells, an increase in HER internalization was observed in metformin-treated cancer cells (Figs. 2B and 2C; Supplemental Figs. 3 and 4). Metformin-induced depletion of membrane HER was accompanied by a decrease in phosphorylated HER2 or phosphorylated EGFR protein levels (Figs. 2B and 2C; Supplemental Figs. 3 and 4). Thus, our findings suggest that short-term incubation with metformin decreases membrane HER and phosphorylated HER oncogenic signaling pathways.
(A) Schematic representation of cell-surface biotinylation approach used to collect membrane-bound and internalized proteins. Schematic was made using BioRender. (B and C) Western blot analysis of biotinylated membrane; internalized, phosphorylated HER2; phosphorylated EGFR; total HER2; and total EGFR on NCIN87 or A431 cancer cells incubated with 5 mM metformin for 2 h. Western blot at bottom shows membrane HER2, membrane EGFR, total HER2, and total EGFR at 0, 4, 12, and 24 h after removing metformin from cancer cells. Cancer cells were incubated with 5 mM metformin for 2 h and washed with fresh culture medium before protein lysates were collected.
Removal of Metformin Causes Rebound Effects on Cell-Surface HER Density
Our in vitro findings demonstrated that membrane HER decreases rapidly within 2 h after cell incubation with metformin (Fig. 1), becoming markedly lower than that of untreated cells. This abrupt decrease in membrane HER was accompanied by a decrease in membrane-bound antibodies (Fig. 1) and an increase in internalized HER (Fig. 2). We next analyzed membrane HER after metformin withdrawal in time-course immunoblotting experiments on cancer cells treated with metformin for 2 h and then released from treatment through a rapid washout procedure. We observed that the amount of membrane HER increased rapidly after metformin washout as it went back to control levels between 12 and 24 h after drug withdrawal (Figs. 2B and 2C). In conclusion, the removal of metformin restores membrane HER after drug washout.
Preincubation with Metformin Reduces Anti-HER Antibody–Tumor Binding
We showed that metformin decreases membrane HER and membrane-bound anti-HER antibody and enhances total CAV1 (Fig. 1). We next performed immunofluorescence assays to determine the effect of metformin on trastuzumab or panitumumab binding to HER2-positive NCIN87 or EGFR-positive A431 cancer cells, respectively (Supplemental Fig. 5). We examined antibody binding to cancer cells preincubated versus coincubated with metformin.
The preincubation approach was applied to determine whether metformin-induced CAV1 expression could decrease antibody binding to cancer cells. When cancer cells were preincubated with metformin for 2 h, we observed an 8.3-fold or 2.6-fold decrease in antibody binding to NCIN87 or A431 cancer cells, respectively (Fig. 3), compared with control.
Representative fluorescence microscopy images and quantification of immunofluorescence staining of HER2 and EGFR receptors in NCIN87 (A) or A431 (B) cancer cells coincubated or preincubated with metformin. ***P < 0.001, based on Student t test and compared with untreated cells (n = 7–10, mean ± SD). DAPI = 4′,6-diamidino-2-phenylindole.
Because metformin is usually prescribed as a daily dose, we evaluated antibody binding to cancer cells in the continuous presence of metformin (coincubation). Although trastuzumab or panitumumab binding decreased in cancer cells coincubated with metformin, the decrease was substantially lower than before metformin treatment (Fig. 3). In our studies, the antibody bound to NCIN87 or A431 cancer cells was 1.2-fold or 1.5-fold lower, respectively, in a coincubation treatment regimen.
These results demonstrate that a preincubation schedule of cancer cells with metformin highly reduces membrane HER, which in turn decreases anti-HER accumulation in cancer cells.
Acute Metformin Treatment Induces Reversible Depletion in Anti-HER Antibody–Tumor Binding
The preceding in vitro data provided the rationale for preclinical imaging studies to annotate trastuzumab and panitumumab tumor binding in mice treated with metformin. In our studies, trastuzumab or panitumumab was conjugated with the Deferoxamine (DFO) chelator and labeled with 89Zr (Supplemental Figs. 6 and 7). Acute oral administration of metformin was performed 1 d before and at the same time as the radiolabeled antibody. This acute metformin treatment schedule was performed to validate our in vitro findings that the effects of metformin-induced depletion of trastuzumab or panitumumab tumor binding are reversible. The dose of metformin used in our imaging studies, 250 mg/kg for mice, corresponds to a human-equivalent dose of 1,219 mg, which is lower than the maximum recommended daily dose in humans of 2,550 mg/d. Control mice were administered saline instead of metformin in the same volume (Fig. 4A).
(A) Schematic representation of PET imaging in mice bearing tumor xenografts treated with acute or daily dose of metformin. (B) Representative 3-dimensional volume-rendered images of PET at 24 and 72 h after tail vein injection of 89Zr-labeled trastuzumab in mice bearing HER2-positive NCIN87 xenografts. Metformin (250 mg/kg) was orally administered 1 d before and at same time as 89Zr-DFO-trastuzumab (6.66–7.40 MBq, 45–80 μg of protein) in acute-dose cohort. In daily-dose cohort, metformin (200 mg/kg) was injected daily by intraperitoneal administration. Scale bar, percentage injected dose (%ID) per gram of the organ. (C) Biodistribution profile of control, acute-dose, and daily-dose cohorts at 72 h after injection of 89Zr-DFO-trastuzumab. MIP = maximum-intensity projection. Supplemental materials provide full list of organ biodistribution. ***P < 0.001, based on Student t test and compared with control (bars, n = 3, mean ± SD). *P < 0.05, based on Student t test and compared with control (bars, n = 3, mean ± SD). BioD = biodistribution.
HER-targeted PET imaging at 24 h demonstrated a lower accumulation of antibody in tumors from metformin-treated mice than from saline cohorts (Fig. 4B; Supplemental Figs. 8–10). Additional PET imaging of these mice revealed similar antibody–tumor accumulation in mice treated with an acute dose of metformin and in control cohorts at 72 h after injection of the radioimmunoconjugate. Ex vivo biodistribution studies validated our findings from PET imaging at 72 h: 55.08 ± 3.84 %ID/g of tumor for 89Zr-trastuzumab in the saline cohort and 56.43 ± 0.54 %ID/g of tumor for 89Zr-trastuzumab in the metformin cohort (Fig. 4C; Supplemental Fig. 9).
These results indicate that the differences in antibody–tumor uptake in mice treated with an acute dose of metformin are temporal, with decreased uptake in metformin-treated tumors at 24 h and similar uptake values at 72 h after antibody injection versus control.
Daily Administration of Metformin Reduces Anti-HER Accumulation in HER2- and EGFR-Expressing Tumor Xenografts
Because metformin is clinically prescribed once daily, we performed additional immuno-PET studies administering metformin daily before 89Zr-labeled antibody injection. The daily dose used in our preclinical studies (200 mg/kg for mice) was lower than the maximum recommended daily dose in humans. Previous studies using this daily dose of metformin demonstrated a high reduction in membrane receptor density (other than HER) without signs of preclinical toxicity (29). Control cohorts were administered saline instead of metformin (Fig. 4A).
Immuno-PET at 72 h after injection of 89Zr-labeled antibody demonstrated a reduction in antibody–tumor binding compared with the control or acute cohorts (Fig. 4B; Supplemental Figs. 8–10). Ex vivo biodistribution studies at 72 h after radiolabeled trastuzumab injection validated our findings from HER2-targeted PET imaging (Fig. 4C; Supplemental Fig. 9). HER2-positive NCIN87 xenografts of mice treated daily with metformin yielded tumor uptake of 15.01 ± 3.84 %ID/g, lower than that in saline cohorts (55.08 ± 7.54 %ID/g) or NCIN87 tumors from mice treated with an acute dose of metformin (56.43 ± 0.54 %ID/g). Similar results were obtained in tumors of mice treated daily with metformin and imaged with radiolabeled anti-EGFR panitumumab (Supplemental Fig. 10). However, the tumor uptake of a radiolabeled IgG control was low and comparable in mice treated with saline and those with daily administration of metformin (Supplemental Fig. 11).
These results suggest that the reduction in membrane HER by daily treatments with metformin is physiologically significant and clinically relevant.
PET Imaging Allows Monitoring of Cell-Surface HER Rebound After Withdrawal of Metformin
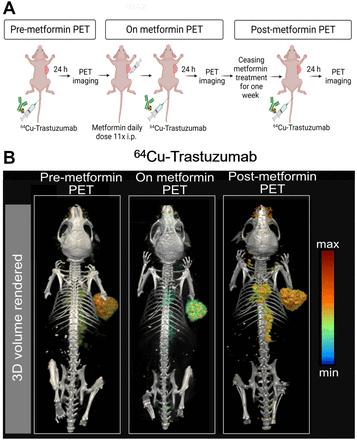
To validate the temporality and reversibility effect of metformin on antibody–tumor binding, we performed PET imaging before, during, and after metformin treatment using trastuzumab labeled with the short-lived isotope 64Cu (Fig. 5A). Mice with HER2-positive NCIN87 xenografts imaged before initiating the metformin treatment demonstrated tumor uptake of 51.54 ± 10.41 %ID/g (Fig. 5B; Supplemental Figs. 12 and 13). PET images of mice treated with a daily dose of metformin showed a 1.8-fold reduction in tumor uptake (27.42 ± 7.5 %ID/g). Finally, PET images of mice at 7 d after stopping the metformin treatment demonstrated tumor uptake similar to that observed before metformin therapy (57.82 ± 15.39 %ID/g). These studies suggest that immuno-PET can effectively track the temporal and reversible changes in antibody–tumor binding as a result of metformin treatment.
(A) Schematic representation of PET imaging before, during, and after treatment with daily dose of metformin. (B) Three-dimensional volume-rendered images of PET acquired at 24 h after injection of 64Cu-trastuzumab. Mice were imaged at 24 h after injection of 64Cu-trastuzumab (6.66–8.3 MBq or 45–56 μg of protein). PET images were acquired at baseline (pre-metformin PET), after daily administration of metformin (on metformin PET), and at 7 d after withdrawal of metformin (post-metformin PET). Scale bar, percentage injected dose (%ID) per gram of the organ.
DISCUSSION
In addition to its antidiabetic properties, metformin induces antitumor effects by directly inhibiting the phosphatidylinositol-3-kinase mammalian-target-of-rapamycin and Ras-mitogen-activated protein kinase (MAPK) pathways (2,4,5,8). The indirect antitumor effects of metformin comprise reduction of glucose, insulin metabolism, and immune responses by regulation of T-cell differentiation and activity (12,29). In addition, metformin reduces cholesterol biosynthesis through its indirect activation of the adenosine monophosphate-activated protein kinase pathway (8,25,26,36,37). Drugs that deplete cholesterol content at cell membranes temporally enhance receptor availability on the surface of cancer cells and tumor xenografts (19–21,28). Here, we show that metformin reduces the surface availability of HER in a time-dependent manner. Metformin reduces membrane HER to decrease antibody binding to tumor xenografts. Our findings highlight the significance of monitoring the effects of over-the-counter medicines in oncology settings.
Treatment with metformin induces HER internalization (Fig. 2), decreasing the number of HER receptors available at the cell membrane for antibody–tumor binding (Fig. 4). A reduction in receptor density at the cell surface has been shown to decrease transphosphorylation, likely by reducing receptor dimerization. Loss of membrane HER in tumors not only decreases HER phosphorylation (Fig. 2) but also reduces antibody–tumor binding in mice treated with a prolonged dose of metformin (Fig. 4).
We found a decrease in the accumulation of trastuzumab or panitumumab binding to tumors as an acute response to oral administration of metformin. The effects induced by acute administration of metformin are reversible: progressive recovery in membrane HER (Figs. 2B and 2C) was detected after removing the metformin from cancer cells, and antibody–tumor accumulation recovered at 72 h in mice treated with only 2 doses of metformin (Fig. 4). However, mice treated daily with metformin showed a greater and more sustained reduction in antibody accumulation in tumors than did control mice or mice treated with an acute dose of metformin. Additional PET imaging studies performed before, during, and after metformin daily treatment demonstrated a rebound of cell-surface HER after withdrawal of the drug (Fig. 5). Because our imaging studies used a full-length antibody, it is possible that blocking of HER2 receptors could occur during the weekly imaging schedule. Therefore, future imaging studies will use a radiolabeled small molecule to monitor the dynamic changes induced by metformin in tumoral HER2. Future studies would be required to determine whether the depletion observed in antibody–tumor binding affects tumor response after treatment with an acute versus a prolonged dose of metformin in the presence or absence of anti-HER therapies.
The therapeutic efficacy of trastuzumab, trastuzumab–drug conjugates (trastuzumab emtansine or trastuzumab deruxtecan), cetuximab, and panitumumab depends on receptor density and HER-antibody trafficking (19–21,38,39). Our previous studies have shown that drugs altering rates of HER endocytosis and recycling result in changes in the surface pool of HER. We have previously reported that cholesterol-depleting drugs (19–21) enhance antibody–tumor binding and efficacy. Others have shown that metformin inhibits transcriptional and translational expression of key components of the cholesterol biosynthetic pathway (8,22–26). These previous reports could indicate that metformin may enhance antibody accumulation in tumors. However, our data suggest that daily administration of metformin reduces trastuzumab or panitumumab accumulation in tumors. Further studies are needed to understand the mechanisms, other than alterations in the cholesterol biosynthetic pathway, that occur in cancer cells treated with metformin.
The CAV1 protein, a major structural protein of cholesterol-rich, caveolae-mediated endocytosis, is involved in HER cell membrane dynamics, and the bioavailability of membrane HER is enhanced via temporal regulation of CAV1 (19–21,27). Others have shown that metformin-induced upregulation of AMP-activated protein kinase increases CAV1 expression in cancer cells, and pretreatment of cancer cells with metformin enhances trastuzumab emtansine efficacy (28,40,41). Here, we provide evidence that an acute dose of metformin upregulates CAV1 at early incubation times and leads to a rebound of membrane HER after the removal of metformin. However, a daily dose of metformin results in sustained depletion of membrane HER and reduces antibody accumulation in tumors. The discovery that metformin-induced depletion in cell-surface HER depends on time and dose may have direct implications for the use of anti-HER antibodies for imaging and therapy. Because many patients with cancer are prescribed metformin, our data provide a rationale to evaluate doses and regimens of metformin treatment that might affect the therapeutic or imaging outcomes in patients with HER2- or EGFR-expressing tumors.
In a recent phase 3 clinical trial (CCTG.MA.32), the addition of metformin to standard adjuvant chemotherapy did not improve progressive-free survival or overall survival for patients with hormone receptor–positive or hormone receptor–negative breast cancer (42). Metformin sensitizes HER-targeted therapies in vitro and in preclinical mouse models of cancer (43,44). The benefits of combining metformin with anti-HER therapies have also been tested in the clinical setting. Although the METTEN prospective clinical study demonstrated that nondiabetic breast cancer metformin users receiving trastuzumab and chemotherapy have a higher pathologic complete response than that of nonusers, the study was underpowered to show synergism (44).
Metformin is administered to humans with type 2 diabetes mellitus orally at therapeutic doses of 500–3,000 mg/d (45). After a single dose, the plasma concentrations peak in 3 h, resulting in concentrations ranging from 10 to 25 μM (46,47). In mice, administering metformin at a 250 mg/kg dose yields plasma concentrations of 125–150 μM after 1–2 h that rapidly decrease thereafter (48). However, metformin treatment of cancer cells requires high doses (in the millimolar range) to exert an anticancer effect or to deplete the cellular abundance of tumor targets. For example, previous studies reported that millimolar concentrations of metformin led to significant depletion of membrane programmed death-ligand 1 or HER (29,49,50). Similar to other studies reporting alterations in membrane receptor density on treatment with metformin (28,29), the in vitro concentrations reported here are higher than the micromolar-range concentrations detected in patients. Despite these differences in metformin dosage in clinical and preclinical settings, recent studies have shown that metformin can significantly reduce the expression of EGFR in clinical samples of oral squamous cell carcinoma (51). Therefore, it is necessary to explore how clinically relevant doses of metformin affect membrane HER and, subsequently, tumor uptake of anti-HER antibodies.
Our study has several limitations. Because the susceptibility of cancer cells to metformin depends on time, dose, and cell line, it is not clear whether the results described here will translate to other tumor types and membrane receptors. Given the ability of metformin to broadly affect cancer cells through multiple pathways, changes in CAV1 and membrane HER may partially explain the ability of metformin to decrease antibody binding in tumors. Although the dosage of metformin used in our in vitro studies is similar to that used in other studies reporting metformin-induced alterations in receptor density, a concentration in the millimolar range is higher than the concentration of metformin detected in serum samples of patients. In addition, the mechanisms by which metformin accumulates in cancer cells and how it confers beneficial effects in patients with cancer are incompletely understood. In our studies, an acute dose of metformin was administered orally to mimic clinical administration, whereas daily administration was performed through intraperitoneal injection with a nontoxic dosage schedule (29). Although intraperitoneal administration of a substance has similar pharmacokinetics to oral administration (52), further clinical studies are necessary to explore whether the route of administration of metformin can affect the uptake or biologic activity of anti-HER antibodies. Furthermore, it is not clear whether the alterations in HER observed in our studies using xenografts will translate into clinical changes due to differences in metformin pharmacokinetics in mice versus humans.
CONCLUSION
Several clinical trials are under way to evaluate metformin for drug repurposing in patients with cancer. Our data suggest that short-term administration (2 d) of metformin treatment results in reversible depletion in membrane HER and, consequently, in temporal depletion of antibody–tumor accumulation. However, daily administration of metformin to mice with HER2-positive or EGFR-positive tumors reduces trastuzumab or panitumumab accumulation, respectively, as a result of reducing target density at the cell membrane. These results suggest that the daily use of metformin may influence the effectiveness of these cancer treatments and imaging outcomes and therefore should be carefully considered in the clinical setting.
DISCLOSURE
This research was supported by internal funds provided by the Mallinckrodt Institute of Radiology (MIR) and in part by the American Cancer Society (IRG-21-133-64-03) and by the National Institutes of Health (NIH) (R37CA276498). Patrícia Pereira is supported by the NIH (R01 CA244233-01A1 and R37CA276498), American Cancer Society (IRG-21-133-64-03), Cancer Research Foundation (P22-03203), Elsa Pardee Foundation, Alvin J. Siteman Cancer Center (SSC) through the Foundation for Barnes-Jewish Hospital, and National Cancer Institute (NCI; P30 CA091842). Abbey Zidel’s and Zachary Fisher’s contributions to the research were supported through Washington University’s biology undergraduate research program (Bio 200/500), MIR Summer Research Program, and the Summer Undergraduate Research Award. The Preclinical Imaging Facility was supported by NIH/NCI SCC support grant P30 CA091842, NIH instrumentation grants S10OD018515 and S10OD030403, and internal funds provided by MIR. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. No other potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: Can HER-targeted antibody PET imaging inform on metformin-induced alterations to the cell-surface density of tumor biomarkers?
PERTINENT FINDINGS: Metformin has both time- and dose-dependent effects on membrane HER density as visualized by immuno-PET.
IMPLICATIONS FOR PATIENT CARE: Metformin-induced downregulation of cell-surface HER reduces the accumulation of therapeutic anti-HER antibodies in tumors that could affect tumor response to therapy.
ACKNOWLEDGMENTS
We thank the Washington University School of Medicine isotope production team for the production of 89Zr and 64Cu, and the small-animal imaging facility for help with the small-animal PET/CT image collection. We thank Dr. Luis Batista for letting us use the Odyssey Infrared Imaging System. We thank Dr. Luke Carter for his help with the 3D Slicer software. We acknowledge the SSC pharmacy for providing us with trastuzumab (Herceptin, Roche) and panitumumab (Vectibix, Amgen) antibodies.
Footnotes
Published online Jun. 2, 2023.
- © 2023 by the Society of Nuclear Medicine and Molecular Imaging.
REFERENCES
- Received for publication November 23, 2022.
- Revision received March 30, 2023.