Visual Abstract
Abstract
Our purpose was to assess response after ablation of thyroid remnants (ATR) with radioactive iodine therapy in patients with unstable Graves’ orbitopathy (GO) after subtotal thyroidectomy. Methods: Thirty patients with mild (n = 4, 13%), moderate-to-severe (n = 25, 83%), or very severe GO (n = 1, 3%) were analyzed in this retrospective study. The primary endpoint was the improvement of GO-related symptoms as assessed by clinical activity scores, NOSPECS, and soft-tissue inflammation scores at 3 and 12 mo after ATR. Ablation success was defined by a decrease in 99mTc uptake on thyroid scintigraphy, remnant volume, and thyrotropin receptor antibody levels at 3 mo after ATR. Results: Twelve months after ATR, clinical activity scores, NOSPECS, and soft-tissue inflammation scores showed a significant decrease from 3.4 to 1.3 (P < 0.0001), 5.9 to 4.9 (P = 0.007), and 4.7 to 2.1 (P = 0.0001), respectively. The GO was inactive in 27 of the 30 (90%) patients after 3 mo and in 29 (97%) after 12 mo. No new activation of GO occurred. Remnant volume (1.4 vs. 0.4 cm3, P = <0.0001), mean thyrotropin receptor antibody level titer (19.02 vs. 13.37 IU/L, P < 0.0001), and 99mTc uptake (0.5% vs. 0.1%; n = 12; P = 0.04) decreased significantly until 3 mo after ATR. Discussion: Radioactive iodine therapy after thyroidectomy can successfully ablate residual thyroid remnants, leading to an improvement in GO, a reduction in inflammatory activity, and stabilization of thyroid function. Thus, scintigraphy should be considered for patients with unstable GO after thyroidectomy to rule out thyroid remnants.
Graves’ orbitopathy (GO), the most common extrathyroidal manifestation of Graves’ disease, is a disorder of autoimmune origin. Typically, patients show symptoms of inflammation of the orbital soft tissues, inflammation-triggered fibrosis of the ocular muscles, and adipogenesis (1–3). Autoantibodies against the thyroid-stimulating hormone (TSH) receptor mediate these changes, which stimulate the receptors on orbital fibroblasts. In conjunction with the induction of crosstalk with insulinlike growth factor 1 receptors, this stimulation leads to a cascade of inflammatory conditions (4). Antibodies and autoimmune T cells stimulate orbital fibroblasts to release inflammatory cytokines to produce hyaluronic acid and to differentiate into adipocytes and myofibroblasts (5–9). Consequently, patients experience signs of inflammation (pain, swelling), diplopia (due to fibrosis of the extraocular muscles), and proptosis (due to adipogenesis), which have a serious impact on quality of life (10,11). Most severely afflicted GO patients can have vision loss due to optic nerve compression (12). Despite recent advances in targeted therapy, there is none available in Europe yet. Therefore, current treatment can often reduce symptoms but not prevent the need for rehabilitative surgery (13–16). Management of GO comprises 2 main therapeutic principles: antiinflammatory treatment and reduction of risk factors for deterioration. According to the EUGOGO (European Group on Graves’ Orbitopathy) 2021 guideline, patients with moderate-to-severe GO are treated with immunosuppression by intravenous glucocorticoids alone or in combination with mycophenolate sodium (17). The aim of this antiinflammatory therapy is to temper inflammation and prevent further deterioration. Poor control of thyroid function and high TSH receptor antibody levels can lead to development of new GO or worsening of preexisting GO (18–21). Consequently, rapid achievement of euthyroidism is crucial (8,17,20–22). Hyperthyroidism is treated primarily with antithyroid drugs. Definitive treatment with radioactive iodine ablation or thyroidectomy is performed in cases of relapse or poor thyroid control despite antithyroid drug treatment (13,17,23). The status of GO has an impact on the choice of procedure; thyroidectomy is recommended in the presence of active GO stages, though radioactive iodine might be used with sufficient corticosteroid prophylaxis (17,23,24). Near-total thyroidectomy is performed on patients with Graves’ disease in some cases, even minimally invasively with video assistance (25). Small remnants are left to preserve the recurrent laryngeal nerve. Scintigraphy is not always performed (26). Therefore, ectopic thyroid tissue is sometimes left behind. There is evidence that larger thyroid residues are associated with poorer control of thyroid function, ongoing GO activity, and persistent thyrotropin receptor antibody (TRAb) levels (27,28). In accordance, several studies have shown a higher rate of stable GO and inactivated GO if thyroidectomy is combined with postoperative radioactive iodine therapy (total ablation) (29–31). This beneficial role might be due to complete thyroid-antigen removal, which is associated with a reduction in antigenic stimulation, a drop in antibody levels and cell-mediated immunoreactivity to TSH receptor, and improvement in GO (31,32). To evaluate the benefit of ablation of a significant thyroid remnant in patients with unstable GO and persistent unstable thyroid function in terms of GO activity and severity, we performed an interdisciplinary retrospective study at our tertiary GO referral center.
MATERIALS AND METHODS
Study Population
We searched the institutional database of our EUGOGO tertiary referral center from January 2005 until October 2020 (n = 4,641) for patients who underwent ablation of thyroid remnants (ATR) for persistent or worsening GO and thyroid dysfunction after subtotal thyroidectomy. Only patients with active GO at baseline, comprehensive eye and thyroid examinations before ATR and 3 and 12 mo afterward, elevated TRAb, and significant uptake on baseline thyroid scintigraphy were included. This retrospective study was performed in accordance with the Declaration of Helsinki and was approved by the Ethics Commission of the University of Essen (reference number 17-7542-BO).
Outcome Measures
Primary outcome measures were an improvement in GO-related symptoms as assessed by clinical activity score (CAS), modified NOSPECS score (where N = no symptoms or signs, O = only signs, S = soft-tissue involvement, P = proptosis, E = extraocular muscle involvement, C = corneal involvement, and S = sight loss due to optic nerve compression) (33), and soft-tissue score at 3 and 12 mo after ATR (17).
For this study, a successful ablation was defined as decreases in TRAb, 99mTc uptake, and ultrasound-derived thyroid volume, as well as an increase in levothyroxine dose at 3 mo after ATR.
Clinical Assessment
Eye examinations were performed using a modified EUGOGO case record form and color atlas in a standardized manner (34). All patients were evaluated by a highly trained orthoptist and by 1 of 2 specialized ophthalmologists. Follow-up examinations were done by the same ophthalmologist at 3 and 12 mo after ATR to ensure homogeneity and reproducibility. GO was diagnosed by the presence of typical clinical signs on examination, including slit-lamp biomicroscopy, applanation tonometry, funduscopy, Hertel exophthalmometry, assessment of subjective diplopia, and objective measurement of deviation using the prism cover test and measurement of monocular excursions. GO activity was evaluated using the CAS classification system established by Mourits et al. (35,36). By analysis of personal photos of the patients and patient history concerning double vision and visual acuity, we determined the dynamic of the disease and scored CAS on a scale of up to 10 points at baseline. GO was classified as active if the CAS value was at least 4/10 points. Additionally, the severity of GO (modified NOSPECS) was classified according to the proposed criteria of the EUGOGO, as previously described (33,37). A maximum of 14 NOSPECS points was possible, with no signs of GO classified as 0 points. In addition, we scored the soft-tissue inflammation signs derived from CAS more gradually as follows: spontaneous retrobulbar pain (0–1), upper lid edema (0–2), lower lid edema (0–2), conjunctival injection (0–1), chemosis (0–1), lid redness (0–1), and swelling of the caruncle or plica (0–1). The sum builds the clinical soft-tissue score.
Thyroid examinations were performed or supervised by a board-certified nuclear medicine physician at baseline and 3 mo after radioactive iodine therapy. The examinations included patient history, ultrasound, and a thyroid panel including thyroid hormones and TRAb in all patients. Follow-up 99mTc-pertechnetate thyroid scintigraphy of residual thyroid gland tissue was performed for a subgroup (n = 12).
Radioiodine ATR
The 131I activity was determined with the aim of delivering an absorbed dose of 500 Gy to the thyroid remnants. To this end, 2 different methodologies were used.
In 17 of the 30 patients, a radioactive iodine uptake (RAIU) test was performed, and the treatment activity was calculated using the formula of Musholt et al. (38). If the target dose could be achieved only by use of excessive administered activity (i.e., considerably higher than 500 MBq), 0.09 mg of recombinant human TSH (rhTSH) were administered on each of the 2 d leading up to ATR. This was the case in 8 of 17 patients with an RAIU deemed insufficient (median, 192 h; uptake, 1.6% vs. 7.4% in those who did not receive rhTSH). This approach was favored in mainly later years and in patients with larger thyroid remnants.
In 13 of the 30 patients, rhTSH was administered as described above, and 99mTc-pertechnetate thyroid scintigraphy performed on the day of the second injection. If the 99mTc uptake was deemed sufficient by the treating physician, the administered dose was calculated as follows, estimating an RAIU of 10%:
The second approach was used mainly in earlier years and in patients with small thyroid remnants, for which the reliability of an RAIU test was considered questionable. Levothyroxine treatment was not withdrawn. ATR was performed with an average activity of 452 MBq of 131I, and measurements of intratherapeutic RAIU were performed twice daily for a minimum of 5 total measurements. These measurements were used to calculate the thyroid remnant doses reached following the MIRD approach. Additionally, 25 patients received oral glucocorticoid therapy with 30 mg of prednisolone for 4 wk. In 5 patients with highly active GO, intravenous glucocorticoid therapy was necessary. After ATR, thyroid parameters were closely monitored, and medication was adapted to ensure normal TSH levels.
Statistical Evaluation
For metric data, median (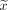 ) and range or mean ± SD were calculated, and differences between groups were evaluated with the Student t test (2-tailed) if the D’Agostino–Pearson omnibus normality test showed a normal distribution or with the Wilcoxon test if the distribution was not normal. The Fisher exact test was used to evaluate group distributions of binary variables. The level of statistical significance was defined as a 2-tailed 2α value of less than 0.05. All calculations were performed with SPSS (version 22.0.0; IBM) and Prism (version 9.0.0; GraphPad) for Windows (Microsoft). P values are given descriptively without α-adjustment for multiple testing.
) and range or mean ± SD were calculated, and differences between groups were evaluated with the Student t test (2-tailed) if the D’Agostino–Pearson omnibus normality test showed a normal distribution or with the Wilcoxon test if the distribution was not normal. The Fisher exact test was used to evaluate group distributions of binary variables. The level of statistical significance was defined as a 2-tailed 2α value of less than 0.05. All calculations were performed with SPSS (version 22.0.0; IBM) and Prism (version 9.0.0; GraphPad) for Windows (Microsoft). P values are given descriptively without α-adjustment for multiple testing.
RESULTS
Study Population
Thirty patients who met all inclusion and exclusion criteria were identified and analyzed. Four showed mild (13%), 25 moderate-to-severe (83%), and 1 sight-threatening (3%) GO. Of these, 27 were female and 3 male; the mean age was 52.1 y (range, 29–80 y) (an overview of baseline characteristics is provided in Table 1). All patients showed unstable GO and fluctuating thyroid parameters. To increase radioiodine uptake, 21 patients received rhTSH before ATR.
Characteristics of Study Population
Changes in Thyroid Parameters
Half the patients (n = 15) showed a remaining pyramidal lobe on pre-ATR 99mTc-pertechnetate thyroid scintigraphy. Because scintigraphy was not routinely included in follow-up examinations, only 6 of 15 patients with a pyramidal lobe received a second analysis after ATR. In all 6 of these patients, the pyramidal lobe was not visible on repeat thyroid scintigraphy 6 mo after ATR (Fig. 1).
Patient example of remaining active thyroid tissue seen on 99mTc-pertechnetate scintigraphy (left) and no uptake 3 mo after ATR (right).
Mean 99mTc uptake in all patients with pre- and post-ATR 99mTc-pertechnetate thyroid scintigraphy decreased from 0.5% to 0.1% at 3 mo after ATR (n = 12, P = 0.04; Fig. 2).
Significant decrease in TRAb level, thyroid volume, and uptake on 99mTc-pertechnetate scintigraphy at 3-mo follow-up compared with baseline, indicating successful ATR. *P ≤ 0.05. ****P ≤ 0.0001. TcTu = 99mTc uptake.
The average thyroid volume (n = 28) shrank from 1.4 to 0.4 cm3 from baseline to 3 mo after ATR. The mean TRAb titer (n = 21) decreased from 19.02 to 13.37 IU/L. Both changes were highly statistically significant (P < 0.0001). All patients showed a positive TRAb titer at baseline. In no case could a complete regression in antibodies be measured after ATR.
Compared with baseline, average TSH increased from 1.3 to 1.42 mU/L (P = 0.75), despite increasing levothyroxine therapy (81.5 vs. 101.3 μg after 3 mo [P = 0.002] and vs. 108 μg after 12 mo [P = 0.006]), emphasizing the loss of functional thyroid tissue and the success of ATR. An overview of the assessed thyroid parameters before and after ATR is provided in Table 2, and an overview of the radioactive iodine therapy parameter is provided in Table 3.
Thyroid Status
Radioactive Iodine Therapy Parameters
Ophthalmologic Assessment
Three months after ATR, CAS decreased significantly from an average of 3.4 to 1.9 (P = 0.0003; Fig. 3). The rate of active forms decreased to 10%. To reach an inactive status, 3 patients needed glucocorticoids in addition to the glucocorticoids all patients received during ablation. After 12 mo, 96% of patients had an inactive status. CAS further improved significantly to an average of 1.3 (P < 0.0001). Worsening of CAS was observed in only 1 patient (4%), who was a heavy smoker and showed unstable thyroid function and high levels of TRAb before ATR.
Significant decrease in disease activity as assessed with soft-tissue score and CAS at 3 and 12 mo after ATR, compared with baseline. NOSPECS also showed significant decrease (P = 0.013) at both time points. Proptosis was mostly stable, with no significant improvement. *P ≤ 0.05. **P ≤ 0.01. ***P ≤ 0.001. ****P ≤ 0.0001; ns = not statistically significant.
The soft-tissue score decreased at 3 mo after ATR to an average of 3.4 (P = 0.002). After 12 mo, there was a highly significant improvement to an average score of 2.1 (P = 0.0001).
NOSPECS was reduced from 5.9 at baseline to 5.2 at 3 mo (P = 0.013; Fig. 3). A significant reduction in NOSPECS was also observed at the evaluation 12 mo after ATR (4.9, P = 0.007). Worsening of NOSPECS at 3 and 12 mo was observed only in the aforementioned high-risk patient.
For the proptosis analysis, we excluded all patients who underwent orbital decompression surgery during the follow-up period and included only patients with clinically significant exophthalmos of at least 20 mm or side differences of at least 2 mm. This left, at baseline, 8 (27%) patients. Three months after ATR, 3 of these 8 patients improved to a clinically significant extent (reduction ≥ 2 mm), 4 patients were stable, and the condition of 1 patient had deteriorated (increase ≥ 1 mm). This was the same at the 12-mo follow-up. All patients whose proptosis was inconspicuous at baseline (n = 16) underwent no changes in proptosis during follow-up.
An improvement or worsening of ocular motility was defined by an increase of reduction in total motility by no less than 8°. Patients who underwent orbital decompression or eye muscle surgery during the follow-up period were excluded, leaving 19 patients for analysis (63%). At the 3-mo follow-up visit, 26% of these patients had improved motility, 74% had stable motility, and 2 patients had decreased motility. Changes in ophthalmologic parameters are shown in Table 4.
Ophthalmic Status
DISCUSSION
The results of this retrospective study show a clinical benefit of ATR in patients with unstable GO after prior thyroidectomy. Because of the complicated anatomic location, small remnants of thyroid tissue can persist after surgery and subsequently trigger hyperthyroidism and GO (39,40). Our results encourage the use of ATR in these patients with unstable thyroid function and, consequently, unstable GO. This practice is in concordance with the therapeutic principle of aiming for stable euthyroidism in GO (11,41).
Ophthalmologic Assessment
Corresponding to the improvement in thyroid parameters, an early response assessment at 3 mo after ATR already showed significant reductions in CAS, NOSPECS, and soft-tissue scores, with further improvements occurring until the 12-mo follow-up. Worsening can mostly be prevented with concomitant glucocorticoid treatment (oral or intravenous, depending on the activity and severity of GO before ATR). Only 1 patient showed worsening of CAS and NOSPECS during the follow-up trial. This individual was a heavy smoker (30 cigarettes per day), which might be the reason for the insufficient treatment response. The significant reduction in inflammatory activity is less likely due to the corticosteroid treatment that patients received during ATR because of the brevity of the treatment (4–6 wk) and previous unsuccessful attempts to stabilize the GO with corticosteroids. However, a beneficial effect cannot be ruled out and should be investigated.
Our findings are in line with the results of prior studies. A retrospective analysis of 55 GO patients who underwent thyroidectomy showed that the prevalence of inactive GO was significantly higher in the fraction of patients treated with additional adjuvant ATR. This has been confirmed in subsequently performed randomized control trials comparing the effects of thyroidectomy versus thyroidectomy plus ATR on GO improvement (29,30). Different results have been reported from a longitudinal study on 60 patients with mild to moderate GO undergoing thyroidectomy, thyroidectomy plus radioactive iodine therapy, or treatment with antithyroid drugs, without statistically significant differences between the thyroidectomy group and the thyroidectomy–plus–radioactive iodine group. Still, both groups showed significantly better outcomes than the group treated with antithyroid drugs (42). The differing results might indicate that not all patients benefit from ATR after thyroidectomy. At our center, therefore, ATR was performed not immediately after thyroidectomy but in cases of unstable GO after surgery, which entails a negative preselector. Our findings therefore indicate a potential role for ATR in this setting as well. In contrast to Menconi et al. (29) and De Bellis et al. (42), we also included patients with severe GO, and in contrast to Moleti et al. (30), intravenous glucocorticoids were administered to only 5 patients.
Despite the large number of GO patients in our tertiary referral center, only relatively few patients could be included in this trial. There were multiple reasons, such as the high number of mild-GO cases and the many externally performed ATR due to the tertiary referral status of our center and the long journeys to it. Furthermore, further treatment after thyroidectomy was only deemed necessary in a small fraction of patients, suggesting that surgery alone may be sufficient in most cases.
Most patients in our analysis showed a moderate-to-severe GO, probably because patients with mild cases are less often referred to a university eye hospital. The higher number of referred cases of more severe GO may also indicate that mild forms need extensive thyroid treatment less frequently. However, this possibility cannot be extrapolated from our data and should be investigated in a larger study. Our cohort had only 1 patient with sight-threatening disease; such patients are rare even in a tertiary referral center.
The less beneficial effect on proptosis and motility was not unexpected, since fibrotic changes in the extraocular muscles and proptosis due to adipogenesis react less to inactivation of GO, as demonstrated in clinical trials of antiinflammatory agents (16,17).
Changes in Thyroid Parameters
In line with prior observations, ATR showed a good safety profile, and the administration of rhTSH was not associated with any severe ophthalmologic side effects (30). Follow-up examinations after 3 and 12 mo showed reductions in TRAb titer, thyroid volume, and 99mTc uptake, implicating successful irradiation of thyroid remnant tissue. We also observed increases in levothyroxine requirements, which may be interpreted as an additional marker of successful ablation but may also be attributable to other causes, such as weight gain, which is commonly observed in patients treated with corticosteroids.
The therapeutic effect of ATR affirms prior observations that even small thyroid remnants can play a role in mediating GO and that its irradiation reduces autoimmune activity (43,44). Of note, half our patients displayed a prominent pyramidal lobe on pretreatment 99mTc scintigraphy, suggesting that its nonresection may play a role in the course of postthyroidectomy hyperthyroidism and GO. However, since 99mTc scintigraphy is not performed as a routine follow-up examination after thyroidectomy, it remains unknown how many patients with residual pyramidal lobes may experience no complications.
Regarding TRAb, most patients (93%) showed levels above 2 IU/L (median, 18.4 IU/L) at baseline 2 y after the beginning of the thyroid disease. This finding agrees with previous studies showing that patients with severe, progressive GO have a persistent TRAb level of at least 2–6 IU/L even 2 y after the onset of Graves’ disease. In contrast, less severely afflicted patients already showed negative TRAb levels at this time point (8,21). Our patient cohort confirms these findings and represents an at-risk cohort regarding the progression of GO. Still, ATR was able to reduce TRAb levels from a median of 18.4 to 13.4 IU/L after 3 mo. Furthermore, the fact that 96% of patients showed inactivation after ATR, even in this at-risk group regarding TRAb levels, underlines the effectiveness of ATR.
Lastly, a wide range of thyroid remnant doses was reached in our cohort. Interestingly, for all patients in whom the thyroid remnant dose of 500 Gy was exceeded by 20% or more, the activity calculation was performed using the pretherapeutic 99mTc-pertechnetate thyroid scintigraphy or rhTSH was administered after the RAIU test, meaning that the conditions during TRAb and RAIU testing were not comparable.
Patients in whom the target thyroid remnant dose was undershot by 20% or more (n = 10) had a lower RAIU during TRAb testing than in the previously performed RAIU test (n = 7) or than the assumed RAIU of 10% in the patients for whom no RAIU test was performed (n = 3).
Limitations
Limitations of this study include its retrospective design and the lack of a control group in which the course of GO without additional ATR could be observed. Additionally, follow-up thyroid scintigraphy was missing in a subgroup of patients. Furthermore, the treatment protocol within the collective was variable, with some patients being treated after an RAIU test whereas in others an RAIU of 10% was assumed if the 99mTc uptake was rated sufficient by visual assessment. On the basis of the small sample size, caution is warranted in comparing these 2 approaches. Yet, insufficient thyroid remnant doses were more commonly observed in the cohort that underwent a pretherapeutic RAIU test (7/17 vs. 3/13), implying that a clear benefit of an RAIU test cannot be stated.
CONCLUSION
Our data indicate that radioiodine ATR is a viable treatment option in patients with unstable GO and persistence of thyroid dysfunction after thyroidectomy. Therefore, scintigraphy should be considered for patients with unstable GO after thyroidectomy and fluctuating thyroid parameters, and additional ablation should be performed if there is a significant thyroid remnant. Persistence of the pyramidal lobe after surgery may play a pivotal role in the pathogenesis. Further randomized, controlled studies are needed to determine the standalone impact of ATR.
DISCLOSURE
No potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: How does thyroid remnant ablation by use of radioactive iodine impact the course of disease in patients with persistent or worsening GO after prior thyroidectomy?
PERTINENT FINDINGS: Ophthalmologic assessment revealed clinical improvement at 3 and 12 mo after thyroid remnant ablation in 29 of 30 patients. Furthermore, ultrasound, thyroid scintigraphy, and TRAb levels indicated successful ablation.
IMPLICATIONS FOR PATIENT CARE: Thyroid remnant ablation is well tolerated and may improve the treatment outcome in GO patients.
ACKNOWLEDGMENT
We would like to pay tribute to our colleague Ina Binse, who contributed to the study design and supervised data collection and analysis but who sadly died before the study was finished.
Footnotes
Published online Nov. 23, 2022.
- © 2023 by the Society of Nuclear Medicine and Molecular Imaging.
REFERENCES
- Received for publication July 14, 2022.
- Revision received October 8, 2022.











