Abstract
241256
Introduction: Treatments involving immune checkpoint inhibitors (ICPI) can be of great benefit for patients with advanced non-small cell lung cancer (NSCLC), yet treatment response is difficult to predict. In addition to PD-L1 expression, the predictive value of several biomarkers has been explored, including promising PET biomarkers such as Total Metabolic Tumor Volume (TMTV). We developed and validated a multivariable model combining baseline (i.e. before ICPI) PET features with clinical and/or biological biomarkers already identified in the literature, and determined whether the resulting score could increase the accuracy in predicting overall survival (OS) for advanced NSCLC treated with first-line ICPI, compared to PD-L1 expression or TMTV alone.
Methods: A retrospective and monocentric training database of 197 patients with advanced NSCLC (no EGFR mutant, nor ALK rearranged tumors, ineligible to surgery) treated with first-line ICPI either alone or in combination with chemotherapy was collected. All metabolically active lesions on baseline [18F]FDG-PET images were segmented (threshold = 4 SUV) and analyzed using LIFEx software [Nioche et al. Cancer Res. 2018]. Twelve PET features, and 5 clinical features (age, ECOG performance status, sex, chemotherapy or not, PD-L1 expression) were included in the analysis. A Cox multivariable model was designed using a backward stepwise model selection, yielding a so-called NSCLC Pro score for each patient. A second dataset of 54 advanced NSCLC patients treated with first-line ICPI from a different center was used for external validation. Performances were evaluated using a Kaplan-Meier analysis and log-rank test. In addition, the 1-year and 2-year OS rates for low- and high-risk patients identified by the NSCLC Pro Score were calculated, using a cut-off value that maximized the log-rank test statistic for OS based on the training database only.
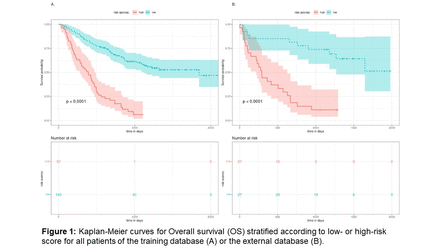
Results: The final multivariable model included 6 features: patient age, TMTV, the Total Metabolic Tumor Volume of pleural metastatic lesions, spleen-to-liver uptake ratio, maximum distance between lesions (SDmax) reflecting lesion dissemination, and the standard deviation of sphericity across all lesions. The model separated patients into low-risk (140 patients) and high-risk groups (57 patients) in the training database, with a significant difference in survival between the two groups (log-rank test: p<0.001, Figure 1). The 1 year-OS rates were 84% for low-risk patients versus 53% for high-risk patients, and the 2 year-OS rates were 68% and 21% respectively. The model showed significantly better performance than TMTV alone or PD-L1 alone (deviance analysis: p<0.001). On the external validation dataset, OS was also significantly different between low-risk (27 patients) and high-risk patients (27 patients, log-rank test: p<0.0001, Figure 1), by using the discrimination cut-off between groups established on the training database. For the external dataset, the 1 year-OS rates were 85% for low-risk patients versus 41% for high-risk patients, and 82% versus 19% at 2 years. The NSCLC Pro Score predicted death at 2 years with a sensitivity and a specificity of 81% on the external database when using the cut-off set on the training data for the identification of low- and high-risk patients.
Conclusions: Using clinical and baseline PET data, we developed a simple and interpretable 6 feature-model that predicts OS in advanced NSCLC patients treated by ICPI. This model was validated on an external cohort from another center without modifying the model parameters or the discrimination threshold between low- and high-risk patients. This model could predict the 2-year survival accurately for more than 80% of patients in the external validation set. NSCLC Pro Score is readily available on the free LIFEx software to allow for external and independent validation.