Abstract
343
Objectives: Synaptic loss is a primary pathology in Alzheimer's disease (AD) and correlates best with cognitive impairment as found in postmortem studies (1). Previously, we observed in vivo synaptic density loss as measured with PET and 11C-UCB-J (radiotracer for synaptic vesicle protein 2A, SV2A) in hippocampus and throughout the cortex (2). Here, we used independent component analysis (ICA), a data-driven method that identifies independent sources (networks) by linearly unmixing the observed signal into maximally independent components. The aim is to apply ICA, which does not use subject group information, to synaptic density PET data to identify brain networks associated with cognitive deficits in AD.
Methods: 11C-PIB PET was performed to determine brain amyloid status. A structural T1-weighted MR was acquired to exclude structural abnormalities and for PET co-registration and analysis. 11C-UCB-J binding to SV2A was measured in 38 AD (24 dementia, 14 mild cognitive impairment) and 19 cognitively normal (CN) participants. 11C-UCB-J distribution volume ratio values were calculated using SRTM2 (population-fixed k2') with whole cerebellum as reference, registered into MNI space and smoothed (8-mm Gaussian). Based on previous SV2A ICA, 18 independent components (IC) were extracted. Subject loadings per IC were compared between groups using unpaired t-tests. Pearson's correlations were used to assess relationships between loading weights and cognitive measures: logical memory II (LMII), Rey auditory verbal learning test (RVLT-delay), clinical dementia rating sum of boxes (CDR-sob), mini-mental state examination (MMSE).
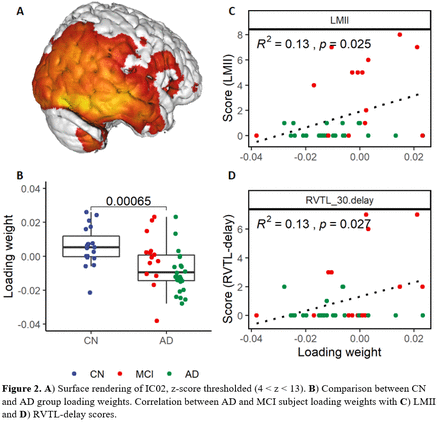
Results: For 8 ICs, we observed significant differences in loading weights between CN and AD groups, including a component (IC01) located in hippocampus and temporal lobe (Fig. 1). Within the AD/MCI group, loading weights for IC02, located in the right parietal-temporal lobe (Fig. 2), correlated with LMII (R2: 0.13, p=0.025), and RVTL-delay (R2: 0.13, p=0.027). For IC03, located in the left parietal hemisphere (Fig. 3), loading weights correlated with CDR-sob (R2: 0.23, p=0.0028) and MMSE (R2: 0.13, p=0.024).
Conclusions: We demonstrated that ICA could define coherent spatial patterns of synaptic density. Furthermore, commonly used cognitive measures correlate significantly with loading weights for two of such networks within only the AD/MCI group. Further studies will explore whether ICA-based networks might be more sensitive than conventional ROI-based analysis. References: (1) Terry RD, Masliah E, Salmon DP, et al. Physical basis of cognitive alterations in alzheimer’s disease: Synapse loss is the major correlate of cognitive impairment. Ann Neurol. 1991;30:572-580. (2) Chen M-K, Mecca AP, Naganawa M, et al. Assessing Synaptic Density in Alzheimer Disease With Synaptic Vesicle Glycoprotein 2A Positron Emission Tomographic Imaging. JAMA Neurol. July 2018.
Participant characteristics and cognitive test scores