Abstract
182
Objectives: Whereas the synthesis of 18F-labeled tracers from aryliodonium precursors and [18F]fluoride ion is well established [1], some precursors generate unwanted and potentially quite difficult to separate regioisomers. Regioisomer formation is likely due to a competing aryne-based mechanism [2], but the circumstances under which this occurs have not been thoroughly investigated. This study investigates regioisomer formation in the radiofluorination of aryliodonium ylides.
Methods: Ylides were synthesized from iodoarenes and Meldrum’s acid [2]. Radiofluorinations were conducted in a modified Synthia apparatus [3] with cyclotron-produced no-carrier added (NCA) [18F]fluoride ion obtained in 18O-enriched water. [18F]Fluoride ion was dried in the presence of cryptand (Kryptofix 2.2.2; K 2.2.2) and base (K2CO3) [4]. A solution of ylide precursor (10 µmol, ~ 15.5 mM) was added to the dried [18F]fluoride ion and heated at 130 °C for 20 min. The reaction was quenched with MeCN: H2O (1:1 v/v, 1 mL) and the crude product was analyzed with reversed phase HPLC. Products were identified by their retention times.
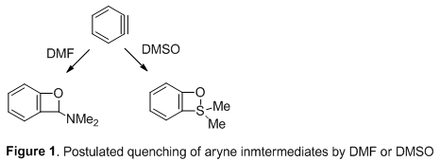
Results: The radiofluorination of 4-methoxyphenyliodonium ylide in MeCN gave average yields of 12% and 5% for [18F]4-fluoroanisole and [18F]3-fluoroanisole, respectively. Conversely, only [18F]4-fluoroanisole (9%, n = 2) was formed when the reaction was conducted in DMF. Inclusion of 1%, 5%, and 10% v/v DMF in MeCN as solvent incrementally decreased regioisomer product from the putative aryne mechanism. Similarly, the reaction in neat DMSO strongly favored the formation of the desired [18F]4-fluoroisomer (40%, n = 2) over [18F]3-regioisomer (<1%). Addition of water, proportionately decreased the yields of both [18F]4-fluoroanisole and [18F]3-fluoroanisole. Conclusions: DMF appears to be effective for suppressing the aryne mechanism and regioisomer formation. We postulate that DMF may form a [2+2] cycloaddition-like adduct [5] with any generated aryne intermediate, thereby preventing reaction of aryne with free [18F]fluoride ion. Similarly, DMSO may form adducts with arynes [6], and this may account for the high regioslectivity for radiofluorination in this solvent. In addition, electronic effects and aryl substituent position in the ylide appear to influence the formation of regioisomers. Further investigations are continuing. Acknowledgements: The Intramural Research Training Award (IRTA) program and the NIH Intramural Research Program (NIMH). References: [1] Pike VW. J. Label. Compd. Radiopharm. 2017; DOI:10.1002/jlcr.3570. [2] Cardinale J, Ermert J, Himpert S, Coenen HH. RSC Adv., 2014, 4, 17293. [3] Bjurling P, Reineck R, Westerburg G, Gee AD, Sutcliffe J, Långström B. Proc. Sixth Workshop on Targetry and Target Chem. Link JM, Ruth TJ (Eds). TRIUMF, Vancouver, 1995, 282 [4] Chun J-H, Lu S, Lee Y-S, Pike VW. J. Org. Chem., 2010, 75, 3332. [5] Yoshioka E, Miyabe H. Tetrahedron, 2012, 68, 179. [6] Li H-Y, Xing L-J, Lo M-M, Wang H, Liu RH, Wang B. Org. Lett., 2015, 17, 1098.