Visual Abstract
Abstract
Radiopharmaceutical therapies (RPTs) based on fibroblast activation protein (FAP) and FAP inhibitors (FAPIs) are a new option for progressive metastatic cancer in patients pretreated multiple times. To date, published in-human data refer to initial experiences with β-emitting 90Y- and 177Lu-based RPT. However, the short tumor retention time of FAPI ligands is considered a major limitation of FAPI RPT. Therefore, fractionated FAPI RPT with 213Bi, an α-emitter with a half-life of 46 min, appears to be a promising FAPI RPT regimen. Here, we report on our initial experiences with regard to the feasibility, tolerability, and response of fractionated 213Bi-FAPI-46 RPT. Methods: Six patients (4 women and 2 men) with progressive metastatic solid tumors (3 colon cancer, 1 anal cancer, 1 breast cancer, and 1 prostate cancer) aged 16–77 y were treated with a mean of 1,609 MBq of 213Bi-FAPI-46, fractionated into 53 single applications (range, 5–12 RPT applications per patient; mean, 8.8 applications) over a period of up to 107 h per patient. Of the 6 patients, 4 patients received adjuvant treatment with pembrolizumab. 18F-FDG (4 patients) and 68Ga-FAPI-46 (5 patients) PET/CT scans were performed before and after RPT. PET images were assessed visually and by calculating total lesion glycolysis and total lesion FAPI. Results: RPT with 213Bi-FAPI-46 was well tolerated without adverse side effects. In terms of visual response assessment, there was 1 partial response (16.7%), 1 patient with stable disease (16.7%), and 4 patients with progressive disease (66.7%). Concordantly, total lesion glycolysis and total lesion FAPI were decreased in the responding patient (not applicable and −24.3%, respectively), slightly decreased in the patient with stable disease (−10.6% and −5.9%, respectively), and increased in the 4 patients with progression (mean, +104.4% and +321.3%, respectively). Conclusion: Fractionated FAPI RPT with the short-half-life α-emitter 213Bi-FAPI-46 is a promising approach that matches the pharmacokinetics of FAPI-46 better than the 177Lu- or 90Y-labeled compounds. In this pilot project, fractionated RPT with 213Bi-FAPI-46 showed good clinical tolerability and even led to regressive or stable disease in the short term in 2 of 6 patients. Further studies with larger patient cohorts are required to evaluate the actual efficacy and long-term effects of this variant of FAPI RPT.
Radiopharmaceuticals based on fibroblast activation protein inhibitors (FAPIs) were introduced in 2018 (1,2), and PET with 68Ga- or 18F-labeled FAPIs has broadly affected oncologic and nononcologic medical imaging (3–6). Next to diagnostic purposes, FAPI tracers represent a new theranostic opportunity for the treatment of a large variety of FAP-positive cancers because of the important role of cancer-associated fibroblasts in the tumor microenvironment, tumor growth, and tumor integrity. In particular, the DOTA-containing FAPI variants (FAPI-02, FAPI-04, and FAPI-46) can be labeled not only with 68Ga but also with therapeutic β−-emitters such as 177Lu and 90Y (1,2) and the α-emitter 225Ac (7), which has shown promising preclinical results in vivo using prostate-specific membrane antigen (PSMA) and DOTATOC ligands (8). Internal radiotherapy with 90Y-labeled FAPI-46 of sarcomas and solitary fibrous tumors has been proven feasible and safe (9–12). However, the efficacy of FAPI therapy is limited by the pharmacokinetics of FAPI-46. Although FAPI-46 has shown prolonged tumor retention compared with previous FAPI variants (FAPI-02 and FAPI-04) (13), the tumor retention of FAPI-46 is still shorter than that of clinically established theranostic compounds such as DOTATOC, DOTATATE, or PSMA ligands. From current experience with radiopharmaceutical therapy (RPT) based on FAPI, modifications such as the use of radionuclides with a shorter half-life, treatment intensification, or hypofractionation and the use of α-emitters appear promising to optimize the efficacy of the treatment.
In this pilot project, we applied hypofractionated FAPI-46 RPT using the α-emitter 213Bi in 6 patients with progressive end-stage cancer to assess the feasibility and safety, as well as the preliminary therapeutic effects, of this variant of FAPI RPT.
MATERIALS AND METHODS
Ethics Approval
All procedures performed in studies involving human participants conformed to the ethical standards of the institutional and national research committees and to the Declaration of Helsinki (1964) and its later amendments or comparable ethical standards. This retrospective study was approved by the local advisory ethic committee (study number S-115/2020).
Patient Selection and Follow-up
Six patients (4 women and 2 men) with advanced-stage cancer after exhaustion of established treatment options were selected as candidates for experimental 213Bi-FAPI RPT by their treating oncologists and gave informed consent to this therapeutic regimen. Detailed information about the types of cancer and previous therapies are presented in Table 1. Four of 6 patients additionally received immunotherapy with the immune checkpoint inhibitor pembrolizumab at 200 mg, administered intravenously, every 3 wk. This humanized monoclonal antibody against programmed cell death protein-1 is frequently used in the treatment of many types of cancer with overexpression of this protein. Immunotherapy with pembrolizumab was administered by the treating oncologists independently from FAPI RPT. Supplemental Figure 1 shows treatment schedules that illustrate the sequence of immunotherapy and FAPI RPT (supplemental materials are available at http://jnm.snmjournals.org).
Characteristics of 6 End-Stage Cancer Patients Treated with Fractionated 213Bi-FAPI-46 RPT
Precursor FAPI-46 and Reagents
The precursor FAPI-46 was synthesized as previously described (13). Solvents and nonradioactive reagents were acquired from B. Braun, Chem-Lab, and Merck and were of Ultrapure, Suprapur, European Pharmacopoeia, or ad iniectabilia grade.
213Bi Radiolabeling of FAPI-46
213Bi could be eluted every 2–3 h from a 225Ac/213Bi generator (ITG Isotope Technologies Garching). For each synthesis, the nuclide was obtained with 1 mL of a freshly prepared 0.1 M hydrochloric acid and 0.1 M sodium iodide solution (14). The eluate (220–350 MBq of 213Bi) was added to 15 nmol of the precursor FAPI-46 in 160 µL of 2.5 mM sodium acetate and 10 µL of 20% ascorbic acid. After the pH was adjusted to a value of 5, the mixture was heated at 95°C for 7 min. Before further processing, the labeling efficiency was assessed by reversed-phase high-performance liquid chromatography (using a linear gradient of 0%–100% acetonitrile in 5 min at a flow rate of 2 mL/min) on a monolithic silica column (Chromolith Performance RP-18e; Merck). The reaction mixture was diluted with 2 mL of isotonic (0.9%) sodium chloride solution (B. Braun) and passed through a preconditioned (1 mL of ethanol followed by 5 mL of 0.9% sodium chloride) Oasis hydrophilic–lipophilic balanced plus light reversed-phase extraction cartridge (Waters). After washing the cartridge with 3 mL of 0.9% sodium chloride, the labeled product was eluted with 1 mL of 70% (v/v) ethanol and 5 mL of 0.9% sodium chloride containing 15 µL of 20% (w/w) ascorbic acid. Between these, 200 µL of 0.6 M sodium phosphate concentrate (B. Braun) was added to achieve approximately pH 7. The final formulation was passed directly through a 0.22-µm sterile filter (Filtropur S 0.2; Sarstedt). Its radiochemical purity was determined by reversed-phase high-performance liquid chromatography and instant thin-layer chromatography. For the instant thin-layer chromatography analysis, an aliquot of the 213Bi-labeled product was diluted 1:1 with 3.8 mM diethylenetriaminepentaacetic acid. Silica gel–impregnated glass microfiber paper strips (Agilent Technologies) served as the stationary phase, and 0.5 M sodium citrate (pH 5) served as the mobile phase.
Fractionated Application of 213Bi-FAPI-46
For safety reasons and because of the reduced clinical condition of the patients, fractionated RPT with 213Bi-FAPI-46 was performed as an inpatient treatment. Vital signs and laboratory parameters (complete blood count, electrolytes, liver and kidney function, tumor markers, etc.) were monitored before and after RPT. We treated the patients with a mean of 1,609 MBq of intravenously administered 213Bi-FAPI-46, fractionated into 53 single applications (range, 5–12 RPT applications per patient; mean, 8.8 applications) over a period of up to 107 h per patient. This required a 2-shift system for the radiochemistry staff and the physicians. The number of RPT applications and the total activity of 213Bi-FAPI administered to each patient was individually adapted according to the FAPI-positive tumor volume and the clinical condition of the patients.
Therapy Assessment by 18F-FDG and 68Ga-FAPI-46 PET/CT
The efficacy of 213Bi-FAPI RPT was assessed by 18F-FDG and 68Ga-FAPI-46 PET/CT. PET scans were performed 0–4 d (18F-FDG) and 5–45 d (68Ga-FAPI) before and 14–28 d (18F-FDG) and 27–46 d (68Ga-FAPI) after RPT. Static PET/CT images were acquired 60 min after the application of 278–314 MBq of 18F-FDG or 165–276 MBq of 68Ga-FAPI-46 using a Siemens Biograph mCT scanner as described previously (6). Three patients underwent both types of PET/CT, 1 patient underwent only 18F-FDG PET/CT, and 2 patients underwent only 68Ga-FAPI-46 PET/CT because of reduced general health status. The patient with prostate cancer (patient 5) underwent 68Ga-PSMA PET/CT before 68Ga-FAPI-46 PET/CT, which excluded PSMA RPT as a therapy option because of PSMA-negative tumor manifestations.
Image Evaluation and Statistical Analysis
All 18F-FDG and 68Ga-FAPI PET/CT images were evaluated by 2 experienced nuclear physicians using threshold-based, automated segmentation included in the affinity tool of Hermes software, version 3.0.5 (Hermes Medical Solutions). Segmentation parameters consisted of a threshold for a signal intensity of SUVmax of at least 4.0 and a minimal size for lesions of 1 mL. Automatically segmented tracer accumulations, which were related to renal excretion or had no tumor-typical CT–morphologic correlate, were deleted from the segmented tumor burden and not further assessed. All segmented tumor lesions were classified as primary tumors or metastatic lesions (lymph node, bone, etc.). Total lesion glycolysis (TLG) and total lesion FAPI (TLFAPI) were calculated as previously described (15,16) and used as therapy assessment parameters. Descriptive statistics were performed to present changes of TLG and TLFAPI after RPT with 213Bi-FAPI-46, as a percentage of the initial values before RPT. No statistical tests were performed.
RESULTS
Feasibility and Tolerability
The mean administrated RPT activity of 213Bi-FAPI-46 was 1,609 MBq (range, 925–1,957 MBq) fractionated into 5–12 RPT applications per patient (mean, 8.8 applications). This variant of RPT was feasible but complex because of the multiple applications of 213Bi-FAPI-46 over a period of up to 107 h per patient. All eligible patients could be treated with fractionated 213Bi-FAPI-46 RPT after establishing a 2-shift system for the radiochemistry staff and the nuclear medicine physicians. No adverse clinical events or unexpected laboratory results were recorded within the follow-up period. All patients tolerated fractionated RPT with 213Bi-FAPI-46 well, and none showed changes of their clinical condition.
Response Evaluation
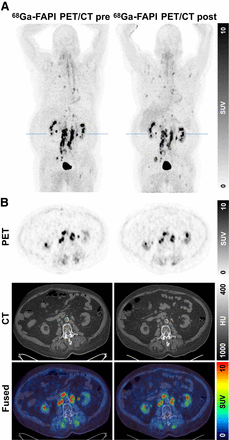
18F-FDG (4 patients) and 68Ga-FAPI (5 patients) PET/CT scans were performed before and after RPT with 213Bi-FAPI-46. In principle, both PET/CT scans showed similar results. On the basis of visual PET/CT image assessment, we observed 1 patient with partial response (16.7%; patient 5), 1 patient with stable disease (16.7%; patient 3), and 4 patients with progressive disease (66.7%) in the follow-up. Concordantly, TLG and TLFAPI were decreased in the responding patient (not applicable and −24.3%, respectively), slightly decreased in the patient with stable disease (−10.6% and −5.9%, respectively), and increased in the other 4 patients with progressive disease (mean, +104.4% and +321.3%, respectively). The increase of TLFAPI in the patients with progressive disease was more pronounced than the corresponding changes based on TLG. As examples, PET/CT images of patient 1 (colon cancer, 18F-FDG vs. 68Ga-FAPI-46) with progressive disease and patient 5 (PSMA-negative prostate cancer, only 68Ga-FAPI-46) with partial response are presented in Figures 1 and 2. Figure 3 shows the calculated values of TLG and TLFAPI of the patients as bar diagrams before and after RPT with 213Bi-FAPI-46, and the changes are displayed as percentages of the initial values.
PET/CT images of 63-y-old male patient with colon cancer and multiple metastases in liver (lower line), lung, lymph nodes, and soft tissue (upper line). (A–F) 18F-FDG (A, C, and E) and 68Ga-FAPI-46 (B, D, and F) PET/CT images before and after 213Bi-FAPI-46 RPT, showing progressive disease. HU = Hounsfield units.
68Ga-FAPI-46 PET/CT images of 77-y-old male patient with PSMA-negative prostate cancer and multiple metastases in lymph nodes and bone before and after 213Bi-FAPI-46 RPT with regressive disease. (A) Maximum intensity projection, with blue line in kidney region. (B) Transversal 68Ga-FAPI-46 PET (top), CT (middle), and fused (bottom) images. HU = Hounsfield units.
TLG (A) and TLFAPI (B) before and after RPT with 213Bi-FAPI-46. Changes of TLG and TLFAPI after RPT are displayed as percentages of initial values, before RPT.
Course of Serum Tumor Markers
All patients presented with at least 1 elevated tumor marker before FAPI RPT. This was mainly carcinoembryonic antigen (patients 1, 3, 4, and 6), followed by cancer antigen 15-3 (patient 2), prostate-specific antigen (patient 5), cancer antigen 19-9 (patient 4), and cancer antigen 72-4 (patient 6). In 4 of 6 patients, the previously mentioned tumor markers were raised after FAPI RPT from +12.9% (patient 5) to +276.3% (patient 6). In the remaining 2 of 6 patients, the tumor markers decreased from −15.7% (patient 1) to −51.4% (patient 4). The course of the previously mentioned tumor markers was heterogeneous and corresponded in only 2 of 6 patients (patients 2 and 6, each with progressive disease) to the visual and metabolic PET/CT response evaluation.
DISCUSSION
FAPI-based RPT represents a potential option in metastasized and pretreated patients with progressive disease after failure of established therapies such as surgery, chemotherapy, or immunotherapy. To date, the published patient series dealing with FAPI RPT comprises different FAPI compounds, for example, FAPI-04 or FAPI-46 labeled with variable radionuclides such as 90Y or 177Lu. FAPI-46 has demonstrated an improved tumor-to-organ ratio with prolonged tumor accumulation compared with FAPI-02 or FAPI-04. Therefore, it was the compound of choice for the present project. Despite the improvements concerning tumor uptake, FAPI-46 shows rapid clearance out of the tumors, which is unfavorable for the use of radionuclides with long half-lives, such as 90Y or 177Lu. On the basis of these properties, an α-emitter with a short half-life and high linear energy transfer, such as 213Bi, could be favorable for FAPI-46 RPT, especially if this variant of RPT is performed in a fractionated manner with multiple applications in each patient.
To date, FAPI-based RPT has been offered only to end-stage patients with poor prognosis after all standard therapies have failed. Therefore, the potential effects of FAPI RPT on local tumor control might be limited to some extent. In particular, because of specific binding to cancer-associated fibroblasts of short-range nuclides, no cross-fire effects on tumor cells can be expected—in contrast to long-range β-emitters such as 90Y. However, targeting of the cancer-associated fibroblasts is still a promising approach given their important role in the tumor microenvironment, as well as the growth and integrity of solid tumors and their metastases. Taken together, FAPI-mediated RPT is an attractive yet not fully understood concept for eligible patients.
Here, we present data that are the first, to our knowledge, on fractionated RPT with 213Bi-FAPI-46 in a small cohort of metastasized end-stage patients with solid tumors. All patients showed progressive disease after established therapeutic options. Therefore, the intention of this treatment was stabilization of the disease as a palliative last-line therapy. Fractionated RPT comprising intravenous applications of 213Bi-FAPI-46 approximately every 10 h was feasible but complex for the patients and the nuclear medicine staff. A time- and staff-intensive 2-shift system was required for the radiochemists and the physicians. No adverse clinical events or unexpected laboratory results were seen within the follow-up period, and all patients tolerated the fractionated RPT well. When RPT with 213Bi-FAPI-46 was taken into account as the last-line option, we found reasonable responses in one third of our small patient cohort. The courses of the tumor markers were heterogeneous and partially inconclusive. We explain these findings mainly by the limited value of tumor markers for the response evaluation of end-stage, potentially dedifferentiated tumors. Nevertheless, there are limitations in our project, such as its retrospective design, the small number of patients treated, the heterogeneous composition of cancer entities, and the short follow-up period because of the limited clinical condition of the patients. However, the primary aim of our pilot project was to prove the feasibility and tolerability of fractionated RPT with 213Bi-FAPI-46, and these questions were adequately addressed. Our results assessing clinical response must be considered preliminary and should be substantiated in larger studies with a more single-entity–focused design.
In general, other published FAPI RPT data or case reports cannot be easily compared with our examination, which included varying types of cancer. In contrast to our patient cohort, most published studies concentrated on sarcoma and pancreatic cancer. In addition, different FAPI compounds with varying radionuclides were used (mostly 90Y and 177Lu), but to our knowledge, no human FAPI RPT study using an α-emitting radionuclide has been published. Nevertheless, some FAPI RPT studies provide an evaluation of the assessed clinical responses and therefore allow some comparison with our data on tolerability and outcome after RPT. Recently, Ferdinandus et al. (10) reported a case series using 90Y-FAPI-46 for RPT in 6 patients with sarcoma and 3 patients with pancreatic cancer. After 1–3 cycles of RPT, the authors observed signs of tumor response in 4 patients with only a few adverse events. In another study, the same group (11) evaluated the safety and efficacy of 1–4 cycles of 90Y-FAPI-46 RPT in patients with advanced sarcoma (n = 16), pancreatic cancer (n = 3), and gastric and prostate cancers (both n = 1). Grade 3 or 4 toxicities, mainly related to the bone marrow, were registered in 8 patients, and 1 partial response and 7 patients with stable disease were noted, especially in the sarcoma cohort. In another RPT pilot study, in which 15 patients with radioiodine-refractory differentiated thyroid cancer were treated with 177Lu-DOTA.SA.FAPI2, 4 patients showed partial response and 3 patients showed stable disease (17). After 45 RPT cycles, no grade 3 or 4 toxicities were observed. In summary, all published external human RPT data describe good tolerability of FAPI RPT, similar to that found in our pilot project, and variable signs of response.
In 2022, Baum et al. (18) published first-in-human data concerning feasibility, toxicity, and dosimetry of 1–3 cycles of RPT using the FAP-binding peptide FAP-2286 labeled with 177Lu in 11 patients (mainly pancreatic and breast cancer) with tolerable side effects, reporting stable disease in 2 patients and progression in 9 patients. Biodistribution images and dosimetric calculations showed high tumor uptake and, in contrast to FAPI compounds, long tumor retention of FAP-2286, which is favorable for labeling with 177Lu. Nevertheless, a comparison of patient outcomes in this FAP study with our FAPI cohort is not possible, because the patient numbers were too small and the entities were too dissimilar. The heterogeneous patient population in both studies underlines the need for future prospective studies on FAP-targeted RPT.
Recently, Cui et al. (19) introduced a highly interesting approach for RPT in general and for RPT using FAPI tracers in particular. A modified variant of FAPI-04 carrying a linker based on sulfur fluoride exchange chemistry dramatically reduced tumor clearance, because the compound was covalently bound to FAP. FAPI RPT using this technique has shown promising preclinical results in a subcutaneous mouse model. If its therapeutic efficacy could be demonstrated in human therapeutic studies, the previously mentioned problems concerning the adverse pharmacokinetics of the original FAPI compounds, which led us to select 213Bi because of its shorter half-life, would be resolved to a certain degree. However, only preliminary in-human data without whole-body images is presented in that study, meaning one can only speculate on the translational relevance of these modified FAPI tracers.
The results of our pilot project with fractionated 213Bi-FAPI-46 RPT in end-stage solid tumors are encouraging and justify further examinations in more patients. 213Bi as an α-emitter with a short half-life and high linear energy transfer could be an advantageous labeling agent for FAPI-46 in a fractionated RPT setting. Furthermore, prospective and randomized clinical trials comparing the different FAPI compounds in homogeneous patient groups are needed to define the most effective RPT variant in terms of optimizing radionuclide and compound selection for eligible cancer types.
CONCLUSION
Our pilot project of fractionated FAPI RPT, with the specific use of the short-half-life α-emitter 213Bi, demonstrates that our approach has potential and is tailored to suit the rapid pharmacokinetics of FAPI-46. Therefore, it could be more effective than other FAPI RPT compounds based on 177Lu or 90Y. Fractionated RPT with 213Bi-FAPI-46 was complex yet feasible, was well tolerated, and resulted in tumor regression or stable disease in one third of our patients with progressive end-stage cancer. On the basis of these promising initial data, further studies with larger patient cohorts are warranted to assess the long-term efficacy of this variant of FAPI RPT. Moreover, prospective and randomized clinical trials comparing the different types of FAPI RPT are required.
DISCLOSURE
This work was funded by the Federal Ministry of Education and Research, grant number 13N 13341. Uwe Haberkorn, Clemens Kratochwil, and Frederik Giesel have filed a patent application for quinoline-based FAP-targeting agents for imaging and therapy in nuclear medicine. Uwe Haberkorn has shares of a consultancy group for iTheranostics. No other potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: Is fractionated 213Bi-FAPI-46 RPT a safe and feasible alternative to RPT with 177Lu- or 90Y-labeled FAPI-46?
PERTINENT FINDINGS: RPT with 213Bi-FAPI-46 was well tolerated without adverse side effects in 6 end-stage cancer patients. We observed 1 partial response, 1 patient with stable disease, and 4 patients with progressive disease.
IMPLICATIONS FOR PATIENT CARE: Fractionated RPT with 213Bi-FAPI-46 can be considered a personalized therapeutic approach for end-stage cancer patients.
ACKNOWLEDGMENTS
We thank our technical and nursing staff for the professional and sensitive care of the patients during imaging diagnostics and FAPI-46 RPT in our department. We also thank Dr. Mark G. MacAskill for final proofreading and language editing of the manuscript.
Footnotes
Published online Oct. 30, 2024.
- © 2024 by the Society of Nuclear Medicine and Molecular Imaging.
REFERENCES
- Received for publication July 10, 2024.
- Accepted for publication September 30, 2024.