Visual Abstract
Abstract
Our rationale was to investigate whether 18F-FDG PET/MRI in addition to (guideline-recommended) conventional staging leads to changes in therapeutic management in patients with newly diagnosed breast cancer and compare the diagnostic accuracy of 18F-FDG PET/MRI with that of conventional staging for determining the Union for International Cancer Control (UICC) stage. Methods: In this prospective, double-center study, 208 women with newly diagnosed, therapy-naïve invasive breast cancer were enrolled in accordance with the inclusion criteria. All patients underwent guideline-recommended conventional staging and whole-body 18F-FDG PET/MRI with a dedicated breast examination. A multidisciplinary tumor board served to determine 2 different therapy recommendations for each patient, one based on conventional staging alone and another based on combined assessment of conventional staging and 18F-FDG PET/MRI examinations. Major changes in therapy recommendations and differences between the conventional staging algorithm and 18F-FDG PET/MRI for determining the correct UICC stage were reported and evaluated. Results: Major changes in therapeutic management based on combined assessment of conventional staging and 18F-FDG PET/MRI were detected in 5 of 208 patients, amounting to changes in therapeutic management in 2.4% (95% CI, 0.78%–5.2%) of the study population. In determining the UICC stage, the guideline-based staging algorithm and 18F-FDG PET/MRI were concordant in 135 of 208 (64.9%; 95% CI, 58%–71.4%) patients. The conventional guideline algorithm correctly determined the UICC stage in 130 of 208 (62.5%; 95% CI, 55.5%–69.1%) patients, and 18F-FDG PET/MRI correctly determined the UICC stage in 170 of 208 (81.9%; 95% CI, 75.8%–86.7%) patients. Conclusion: Despite the diagnostic superiority of 18F-FDG PET/MRI over conventional staging in determining the correct UICC stage, the current (guideline-recommended) conventional staging algorithm is sufficient for adequate therapeutic management of patients with newly diagnosed breast cancer, and 18F-FDG PET/MRI does not have an impact on patient management.
Breast cancer is the most common solid tumor in women worldwide, having an incidence rate higher than 2.3 million in 2020 and being responsible for about 12% of all cancer diagnoses per year (1). Aside from tumor biology, tumor stage is known to be the most important predictive factor regarding the prognosis of breast cancer patients. The 5-y survival probability of cancer limited to the breast amounts to 98.9%, whereas the 5-y survival rate is 85.2% in cases of locoregional metastases and worsens dramatically to 26.9% in cases of distant metastases (2).
Therefore, accurate initial staging in patients with newly diagnosed breast cancer is critical, as this ultimately determines the best treatment plan, can help to avoid potentially harmful and unnecessary surgical interventions, and can markedly influence survival (3). According to current European Society of Medical Oncology (ESMO) and American Society of Oncology guidelines, staging in patients without increased risk focuses on assessing locoregional disease, using mammography, ultrasound, and dynamic contrast-enhanced breast MRI (4). In high-risk patients, additional whole-body imaging is performed using CT, bone scintigraphy, or 18F-FDG PET/CT (5).
PET/MRI has been available since 2010, representing the newest of the hybrid imaging modalities. It combines functional as well as morphologic high-resolution MRI data with metabolic information from PET, providing morphologic imaging datasets with complementary information (6). Especially in the field of breast imaging, the introduction of PET/MRI scanners has opened a new field of research and has shown diagnostic superiority in staging when compared with conventional imaging and PET/CT (7–9). However, data on the potential impact on treatment and long-term patient outcome due to improved diagnostics using PET/MRI are limited (10).
Therefore, the aim of this study was to investigate whether the additional information from 18F-FDG PET/MRI when compared with (guideline-recommended) conventional staging leads to treatment changes in patients with newly diagnosed breast carcinoma and to compare the diagnostic accuracy of 18F-FDG PET/MRI with that of conventional staging for determining the Union for International Cancer Control (UICC) stage.
MATERIALS AND METHODS
Study Design and Participants
This prospective, double-center study was approved by the local ethics committees (study numbers 17-7396-BO and 6040R). Written informed consent was obtained from all patients. The study was performed in accordance with the Declaration of Helsinki. The independent Clinical Trial Centre Essen monitored the study and reviewed safety data and the progress of the study. The study is registered at the German Clinical Trials Register (register number DRKS00005410) and has been funded by the German Research Foundation (BU3075/2-1, BU3075/2-2, and KI2434/1-2).
During recruitment, all consecutive female patients aged 18 y or older presenting to the breast cancer centers at the Universities of Duisburg–Essen and Düsseldorf were prospectively screened to determine whether they met one of the inclusion criteria for an elevated risk of distant metastases: a tumor of stage T2 or higher (detected by conventional imaging), a triple-negative tumor of any size, or a tumor with molecular high-risk features (Ki-67 > 14% or grade 3 or HER2 overexpression).
Exclusion criteria were any other malignancy in the past 5 y, a contraindication to MRI or MRI contrast agents, end-stage kidney disease with a glomerular filtration rate of less than 30 mg/dL, pregnancy or breastfeeding, and the inability to provide informed consent. Furthermore, patients with incomplete or temporally inconsistent imaging datasets were excluded from further data evaluation.
Procedures
The conventional staging procedures for all patients of the study—including x-ray mammography; ultrasound of the breast and axillary fossa; CT of the chest, abdomen, and pelvis; and bone scintigraphy—were performed as part of the clinical routine following the current ESMO guidelines (4). The possibility of an additional PET/CT examination in certain situations was waived because of the routinely planned PET/MRI in view of radiation protection.
All patients also underwent an additional 18F-FDG PET/MRI examination of the breast and whole body. The 18F-FDG PET/MRI examinations were performed at the University Hospital Essen on an integrated 3-T PET/MRI system (Biograph mMR; Siemens Healthcare GmbH). PET/MRI examinations included full diagnostic breast 18F-FDG PET/MRI and whole-body 18F-FDG PET/MRI from head to mid-thighs and were performed with the patient supine and prone according to a previously published, established staging algorithm (11). Image analysis was performed by experienced, board-certified radiologists and nuclear medicine physicians in accordance with current guidelines for breast and whole-body staging. In cases of additional suggestive lesions (e.g., liver or bone lesions) on 18F-FDG PET/MRI and after extensive risk–benefit assessment, tissue sampling was performed by CT- or surgery-guided biopsy for histopathologic verification. Figure 1 shows the study design.
Study design. Histologically proven breast cancer was present in all patients. All patients underwent whole-body 18F-FDG PET/MRI including dedicated 18F-FDG PET/MRI breast examination, as well x-ray mammography; ultrasound of breast and axillary fossa; CT of chest, abdomen, and pelvis; and bone scintigraphy for staging purposes. Multidisciplinary tumor board served to determine 2 different therapy recommendations for each patient, based on conventional staging alone and on combined assessment of conventional staging and 18F-FDG PET/MRI examinations.
UICC tumor stage was determined according to the AJCC Cancer Staging Manual separately for both the conventional staging procedures and the 18F-FDG PET/MRI scans (12). Readers did not know the results of the other imaging. The reference standard for T stage was the expert consent on all available imaging data of the breast, including mammography, ultrasound of the breast, and breast MRI. The routine breast MRI of all patients exceeded the conventional staging recommendations of the actual guidelines. If available, axillary dissection or sentinel lymph node biopsy before systemic therapy was used as a reference standard for N stage. If no sufficient pretherapeutic sampling was available, sentinel lymph node excision or axillary dissection after neoadjuvant systemic therapy was used as a surrogate reference standard (13). The reference standard for distant metastases (M stage) comprised either an imaging follow-up using CT/MRI (n = 5) (in cases of clear distant malignancy due to imaging) or CT-guided or surgical biopsy (n = 10) (in cases of unclear imaging results).
Furthermore, every patient underwent a follow-up whole-body MRI scan 1 y after the initial 18F-FDG PET/MRI examination or clinical follow-up to exclude potential false-negative findings.
Therapeutic Management
Each patient was presented to a multidisciplinary tumor board of the referring gynecology department in clinical routine. All necessary information about tumor biology and patient history was given to the board. Therapeutic management for each patient was determined on the basis of a 2-step process: the first was conventional staging alone, and the second was combined assessment of conventional staging and 18F-FDG PET/MRI, as well as histopathologic results in cases of additional biopsies due to PET/MRI findings. Clinical recommendations for therapeutic management were based on step 2.
Endpoints
Major changes in therapeutic management based on the conventional staging algorithm versus combined assessment of conventional staging and 18F-FDG PET/MRI were defined as the primary endpoint. A major change in therapeutic management was defined as a change from breast-conserving therapy to mastectomy, a change to additional neoadjuvant therapy, a change from sentinel lymph node biopsy to axillary dissection with or without radiotherapy, or a change from curative to palliative care.
The secondary endpoint was defined as comparison of diagnostic accuracy between the conventional staging algorithm and 18F-FDG PET/MRI for definition of the UICC classification. All available information from histopathology, staging procedures, and clinical and imaging follow-up served as the reference standard.
In addition, a subgroup analysis of the different inclusion criteria was performed with respect to the likelihood of the presence of locoregional lymph node metastases or distant metastases.
Statistical Analysis
Because of missing PET/MRI data for breast cancer patients during the planning phase and at the beginning of the study, the sample size was based on a literature-based expected proportion of an 8.1% change from breast-conserving therapy to mastectomy when performing preoperative breast MRI after x-ray mammography (14). The aim was to include 199 patients, enabling detection of a relative frequency of more than 3% (with a power of 90% and 1-sided α of 2.5) for a change in therapy recommendations after additional staging by 18F-FDG PET/MRI.
SAS software (SAS Institute) was used for statistical analysis. Descriptive analysis was performed, and data were presented as mean ± SD.
We calculated and reported CIs to assess the precision of our estimates because our goal was estimation and not significance testing. We wished to avoid publication bias by preferential reporting of significant results. Instead, we judged the value of our estimates by their precision and validity.
RESULTS
In total, 219 patients were enrolled between March 2018 and September 2020, of whom 208 met the inclusion criteria (Fig. 2). Detailed patient characteristics are shown in Table 1, and an overview of performed therapies is in Table 2.
Consolidated Standards of Reporting Trials (CONSORT) diagram.
Demographic Data
Overview of Performed Therapies (n = 208)
Primary Endpoint: Major Changes in Therapeutic Management
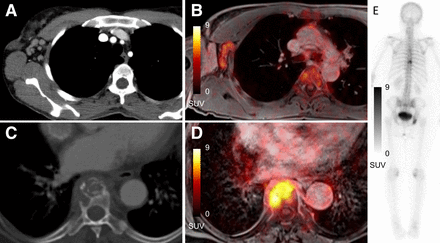
Major changes in therapeutic management based on combined assessment of conventional staging and 18F-FDG PET/MRI were detected in 5 of 208 patients, amounting to changes in therapeutic management in 2.4% (95% CI, 0.78%–5.2%) of the study population. In 3 of those 5 patients, therapeutic management was changed from a primarily curative to a palliative intent (UICC IIIA, UICC IIIA, and UICC IIIB to UICC IV). In 1 case, an additional radiation boost to the supraclavicular fossa was performed because of an additional lymph node metastasis detected on 18F-FDG PET/MRI (UICC IIB to UICC IIIC). Last, breast 18F-FDG PET/MRI helped to correctly downsize the primary tumor in 1 patient (comparison of the assumed size on mammography and ultrasound to PET/MRI: T2 to T1c; UICC IIA to UICC IA), which led to the omission of the primarily intended additional boost of radiotherapy. Figure 3 provides an imaging example.
Comparable diagnostics in conventional staging and 18F-FDG PET/MRI for 47-y-old woman with newly diagnosed breast cancer and locoregional lymph node and bone metastases. Lymph node metastases were seen on CT (A) and T1-weighted MR (B) images with PET fusion with SUVmax of 6.1. Vertebral bone metastasis was seen on CT (C), T1-weighted MR images with PET fusion (D), and bone scan (E) with SUVmax of 8.2.
Secondary Endpoint: Comparison of Diagnostic Accuracy of Conventional Staging Versus 18F-FDG PET/MRI Staging for Determination of UICC Classification
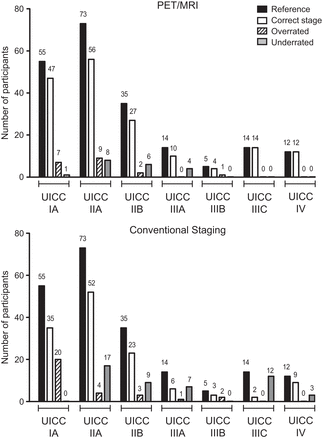
For determination of the UICC stage, the conventional staging algorithm and 18F-FDG PET/MRI were concordant in 135 of 208 patients (64.9%; 95% CI, 58%–71.4%). On the basis of the reference standard, the conventional staging algorithm correctly determined the UICC stage in 130 of 208 (62.5%; 95% CI, 55.5%–69.1%) patients, and 18F-FDG PET/MRI correctly determined the UICC stage in 170 of 208 (81.9%; 95% CI, 75.8%–86.7%) patients. Figure 4 provides an overview of the distribution of UICC stages based on the reference standard and of the corresponding ratings based on conventional staging and 18F-FDG PET/MRI. 18F-FDG PET/MRI yielded false-positive findings in 3 patients (1.4%; 95% CI, 0.3%–4.2%), comprising 2 bone lesions and 1 liver lesion that were proven benign in histopathology.
Distribution of UICC stages based on reference standard and corresponding ratings based on conventional and 18F-FDG PET/MRI staging.
Subgroup Analysis of Different Inclusion Criteria with Respect to Probability of Presence of Locoregional Lymph Node or Distant Metastases
The subgroup analysis of all initially enrolled 219 patients revealed that of the inclusion criteria that are considered associated with an elevated risk of metastasis, tumor size had the strongest influence on metastatic risk. Thus, tumors measuring at least 2 cm in diameter were associated with locoregional lymph node metastases 28.7% (95% CI, 16.2%–41.3%) more often than tumors smaller than 2 cm. Grading had the least influence on the presence of locoregional lymph node metastases, with a difference of 3.9% (95% CI, −9.2% to 17.1%) between the grade 3 and the grade 1/grade 2 subgroups. For the presence of distant metastases, the highest influence was shown for tumor size, with a cutoff at 2 cm resulting in a difference of 5.9% (95% CI, 0.8%–10.9%). The lowest influence was shown for Ki-67 expression, with a difference of 0.4% (95% CI, −11.2% to 12%) between expression of at least 14% and below 14%. Table 3 shows all information for the subgroup analysis.
Subgroup Analysis of Different Inclusion Criteria with Respect to Likelihood of Presence of Locoregional Lymph Node Metastases or Distant Metastases
DISCUSSION
To our knowledge, this was the first prospective double-center study systematically investigating the impact of 18F-FDG PET/MRI on patients with newly diagnosed, therapy-naïve breast cancer and an elevated risk for metastases. Although 18F-FDG PET/MRI staging was superior to conventional staging regarding determination of the UICC stage, the additional information provided by 18F-FDG PET/MRI led to changes in therapeutic management in only a small percentage of patients. Thus, the primary endpoint of this study was not met, indicating no benefit for tumor staging with whole-body 18F-FDG PET/MRI compared with currently recommend staging algorithms when considering patient management.
PET/MRI studies in recent years have focused mainly on comparing the diagnostic accuracy of PET/MRI with other staging modalities such as CT, PET/CT, or MRI (15–17). The diagnostic superiority of 18F-FDG PET/MRI for staging purposes in comparison to conventional staging modalities was also confirmed in our study. However, prospective data on the impact of this diagnostic superiority on therapeutic management have not been available to date. So far, only retrospective PET/CT studies investigating the hypothetic impact on treatment planning have been reported (18–20). These studies found that the 18F-FDG PET/CT imaging resulted in a change in therapeutic management in 8%–18% of patients. These results were not confirmed in this prospective study in a clinical setting as reflected by the decisions of clinical multidisciplinary tumor boards. Hence, despite the apparent difference in diagnostic accuracy between the conventional staging algorithm and 18F-FDG PET/MRI (64.9% vs. 81.9%), the impact on therapeutic management has to be considered minor. One reason may lie in the fact that the treatment of locally advanced breast cancer (≤stage IIIB) is determined primarily by tumor biology and not UICC stage. Although previous studies have shown that multiparametric PET/MRI can provide valuable information about tumor biology, this has not yet had a direct impact on treatment planning in clinical practice (21–23). One other reason may lie in the rationale of a possible selection bias due to the included high-risk breast cancer patients in the present study with a recommendation for neoadjuvant therapy and thus with limited possibilities for changing therapeutic management.
Nevertheless, this study underlined that the conventional staging algorithm tends to underestimate tumor spread, especially in higher stages. The conventional staging algorithm understaged 12 of the 14 patients with advanced stage IIIC (86%) and 3 of the 12 stage IV patients (25%). These patients were correctly staged by 18F-FDG PET/MRI. Nevertheless, the differences in tumor stage determination led to a change in therapy in only a few cases. The initially correct as IIIC classified tumor stage on 18F-FDG PET/MRI led to a change in therapy in only 1 case, compared with the underestimated patients with the conventional algorithm. Here, the radiation field was expanded after successful neoadjuvant therapy because of additionally detected supraclavicular lymph node metastases. In the other patients, the number of additional lymph node metastases in the axilla detected by 18F-FDG PET/MRI had no influence on therapy, as a pathologic complete response was observed after neoadjuvant systemic therapy. Additional bone metastases were detected by 18F-FDG PET/MRI in all 3 patients, resulting in a change in management from primarily curatively intended neoadjuvant to palliative treatment. These findings underline recent studies reporting MRI or 18F-FDG PET/MRI to have higher sensitivity than CT or 18F-FDG PET/CT and bone scintigraphy for bone metastases (24).
The superiority of breast MR over x-ray mammography and the associated influence on treatment planning have been known and well studied (25,26). In our study, therapy changes due to the additional information derived from the dedicated 18F-FDG PET/MRI breast examination occurred in only 1 patient, whereas previous studies described therapy changes in 8.1% when preoperative breast MRI was performed after x-ray mammography (14). The smaller number of therapy alterations in our study is most likely due to the study design. Here, MRI was not investigated against mammography, as in other studies, but against the imaging modalities of the conventional algorithm: mammography and ultrasound.
Overall, the rather minor impact of 18F-FDG PET/MRI on therapeutic management in addition to conventional staging underlines the diagnostic importance and value of the guideline-recommended conventional staging algorithm. Hence, it remains debatable whether the advantages of 18F-FDG PET/MRI, such as reduced radiation exposure and the comprehensive all-in-one staging algorithm as described in previous publications (27–30), justify the significantly higher financial expenditure and the potential risk of additional invasive tissue sampling due to false-positive findings as reported in our study in 1.4% of the cases. Nevertheless, our study showed that on the basis of conventional staging, higher tumor stages in particular are underestimated. Unfortunately, no statement can be made about a cutoff stage in conventional staging at which there is a risk of underestimation. Further studies are needed to determine a threshold for risk underestimation in conventional staging. On the basis of the subgroup analysis, tumor size might be a distributor, as larger tumors with a diameter of at least 2 cm seem to be associated with a higher risk for distant metastases. Thus, 18F-FDG PET/MRI should be considered primarily in patients for whom there is a strong clinical suspicion of distant metastases, as recommended by the 2020 ESMO and the 2019 updated National Comprehensive Cancer Network guidelines.
There are a few limitations that should be mentioned. One is that all patients underwent conventional staging as recommended by the current ESMO guideline, with differences in conventional staging according to National Comprehensive Cancer Network guidelines. In addition, all patients underwent conventional staging according to the current ESMO standards, which differ from the recommendations of the National Comprehensive Cancer Network guidelines and therefore limits clinical transferability. However, lacking an international breast cancer guideline, the study was intended to reflect everyday clinical practice in Europe to assess its direct impacts on diagnostic management. Furthermore, breast sonography was performed by specialized senologists. Even if this German standard deviates from the European standard, a high certification by the German Society for Ultrasound in Medicine ensured a patient-centered highest diagnostic standard in everyday clinical practice. Finally, although our results demonstrate that staging of breast cancer patients with 18F-FDG PET/MRI leads to therapy changes in a small percentage of cases compared with the conventional staging algorithm, the recurrence rate and overall survival were not assessed because of the short follow-up period. This should be the subject of a future analysis.
CONCLUSION
Despite the diagnostic superiority of 18F-FDG PET/MRI over conventional staging in determining the correct UICC stage, the current (guideline-recommended) conventional staging algorithm is sufficient for adequate therapeutic management of patients with newly diagnosed breast cancer, and 18F-FDG PET/MRI does not have an impact on patient management.
DISCLOSURE
The study was funded by Deutsche Forschungsgemeinschaft (DFG), the German Research Foundation (BU3075/2-1, BU3075/2-2, and KI2434/1-2). Wolfgang Fendler reports fees from SOFIE Biosciences (research funding), Janssen (consultant, speakers’ bureau), Calyx (consultant), Bayer (consultant, speakers’ bureau, research funding), Parexel (image review), and AAA (speakers’ bureau) outside the submitted work. Ken Herrmann reports personal fees from Bayer, personal fees and other fees from SOFIE Biosciences, personal fees from SIRTEX, nonfinancial support from ABX, personal fees from Adacap, personal fees from Curium, personal fees from Endocyte, grants and personal fees from BTG, personal fees from IPSEN, personal fees from Siemens Healthineers, personal fees from GE HealthCare, personal fees from Amgen, personal fees from Novartis, personal fees from Y-mAbs, personal fees from Bain Capital, and personal fees from MPM Capital outside the submitted work. No other potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: Does additional 18F-FDG PET/MRI staging lead to changes in therapeutic management and outperform the diagnostic accuracy of conventional (ESMO-guided) staging for determining the UICC stage in patients with newly diagnosed breast cancer?
PERTINENT FINDINGS: In this prospective study, major changes in therapeutic management based on combined assessment of conventional and 18F-FDG PET/MRI staging were detected in 5 of 208 (2.4%) patients. UICC stage was correctly determined by the conventional guideline algorithm in 130 of 208 (62.5%) patients and by 18F-FDG PET/MRI in 170 of 208 (81.9%) patients.
IMPLICATIONS FOR PATIENT CARE: 18F-FDG PET/MRI outperformed conventional (ESMO-guided) staging in determining the correct UICC stage, without an impact on therapeutic patient management.
ACKNOWLEDGMENT
We are grateful for the steady support by Diana Lütke-Brintrup, Working Group Medical Documentation, Institute of Medical Informatics, Biometry and Epidemiology, Medical Faculty, University of Duisburg–Essen, Germany.
Footnotes
Published online Oct. 10, 2024.
- © 2024 by the Society of Nuclear Medicine and Molecular Imaging.
REFERENCES
- Received for publication May 14, 2024.
- Accepted for publication August 8, 2024.