Visual Abstract
Abstract
Prostate-specific membrane antigen (PSMA) is a theranostic target for metastatic prostate cancer (PCa). However, castration-resistant PCa (CRPC) may lose PSMA expression after systemic therapy. Fibroblast activation protein (FAP), expressed by carcinoma-associated fibroblasts in various cancer types, including PCa, has the potential to be an alternative target. In this study, we evaluated FAP expression in CRPC to assess its potential, using PSMA as a comparison. Methods: FAP expression was assessed using immunohistochemistry in 116 CRPC tumors: 78 adenocarcinomas, 11 small cell carcinomas, and 27 anaplastic carcinomas. Correlation analysis between manual scoring and automated scoring was performed on 54 whole-slide sections of metastatic CRPC. Paired FAP and PSMA stains were assessed in tissue microarray cores of CRPC (n = 62), consisting of locally advanced CRPC (n = 9) and metastatic CRPC (n = 53). FAP and PSMA positivity was defined by an immunohistochemistry score of at least 10. To explore the correlation of PSMA and FAP inhibitor (FAPi) PET imaging and immunohistochemistry, a preliminary analysis of 4 patients included in a [68Ga]-FAPi-46 imaging trial (NCT04457232) was conducted. Results: Manual and automated scoring of FAP yielded results with strong correlations. Overall, FAP expression in CRPC was notably lower than PSMA expression (median immunoscores, 14 vs. 72; P < 0.001). Different histologic subtypes of CRPC demonstrated distinct levels of PSMA expression, whereas their FAP expression levels were comparable. Among the 19 PSMA-negative tumors, 11 (58%) exhibited FAP positivity. FAP expression levels in lymph node metastases were significantly lower than those in nonnodal metastases (P = 0.021). Liver metastases showed significant enrichment of tumors with strong FAP expression compared with nonliver lesions (P = 0.016). In the 4 clinical trial patients, the biopsied metastatic lesions showed lower uptake on FAPi PET than on PSMA PET (median SUVmax, 9.6 vs. 14.5), consistent with FAP expression that was lower than PSMA expression in the corresponding tumor biopsy samples (median immunoscores, 30 vs. 160). Conclusion: Because of the low FAP expression levels in CRPC, the utility of FAPi PET imaging may be limited. Although FAPi PET imaging may be further tested in PSMA-negative CRPC, such as small cell carcinoma, other molecular imaging modalities should be evaluated as alternative choices.
Men with localized prostate cancer (PCa) are typically treated with surgery and radiation, whereas those with disseminated PCa are generally managed using systemic therapy, that is, androgen deprivation therapy, androgen receptor inhibitor therapy, and chemotherapy. Theranostic modalities that target prostate-specific membrane antigen (PSMA) have revolutionized PCa management by enhancing disease staging with PSMA PET (1–7) and providing treatment to late-stage patients with [177Lu]PSMA-617 radiopharmaceutical therapy (8). However, PSMA PET may miss up to 15% of clinically significant primary PCa that does not express PSMA (9,10). Furthermore, PSMA PET may miss up to 31% of late-stage PCa, primarily because of tumor transdifferentiation to PSMA-negative tumor subtypes under the selection pressure of androgen-targeted treatments and other systemic therapies (11–14). These transformed PSMA-negative subtypes, including small cell carcinoma (SmCC), are extremely aggressive and associated with a poor prognosis. Therefore, identifying alternative theranostic biomarkers for PSMA-negative PCa represents an unmet clinical need.
Fibroblast activation protein (FAP) is expressed by carcinoma-associated fibroblasts in many solid cancer types, including PCa (15–18). FAP is a type II transmembrane serine protease that is expressed in chronic inflammation, wound healing, and fibrosis. FAP is essentially absent from nonneoplastic tissue and benign tumors. Quinoline-based PET tracers derived from an FAP inhibitor (FAPi) have been developed and represent a promising approach to FAP-targeted imaging for several solid malignancies (16–20). FAP-targeted cancer therapies are in development (21,22). Studies have reported minimal FAP expression in localized PCa and significantly increased FAP expression in late-stage PCa (19,20). FAP may be a potential theranostic biomarker that may complement PSMA imaging and radiopharmaceutical therapy in men with advanced PCa. In this study, we hypothesized that FAP is overexpressed in PSMA-negative castration-resistant PCa (CRPC). We evaluated the expression of FAP and PSMA in CRPC tissue samples, with a focus on FAP expression levels in PSMA-negative tumors. Furthermore, we explored immunohistochemistry and PET imaging correlation in a preliminary analysis of 4 patients included in a prospective exploratory [68Ga]FAPi-46 imaging clinical trial (NCT04457232).
MATERIALS AND METHODS
CASCADE Rapid Autopsy Cohort
Unstained standard whole-slide sections (1 slide per block) were retrieved from 54 CRPC tumor samples from 12 patients enrolled in a community-based rapid autopsy program (CASCADE) described previously (23). The inclusion criterion for this analysis was that the patient died from metastatic CRPC (mCRPC). The CASCADE program was sponsored and conducted by the Peter MacCallum Cancer Centre, Melbourne, Victoria, Australia, and approved by the Human Research Ethics Committee. Tumor samples were obtained after death, and site of biopsy was carefully annotated. All patients provided informed consent. Histologic subclassification was performed by an experienced urologic pathologist based on histomorphology. The CASCADE cohort consisted of 41 adenocarcinomas and 13 anaplastic carcinomas. Anaplastic carcinoma was defined as poorly differentiated carcinoma with significant nuclear pleomorphism or carcinoma with neuroendocrine features but lacking the classic histologic features of SmCC, that is, small, round or spindle cells with scant cytoplasm and absent or inconspicuous nucleoli (24).
Tissue Microarray (TMA) Cohort
In total, 210 TMA cores of CRPC (70 tumors), consisting of surgically resected cases of locally advanced CRPC (n = 14) from Johns Hopkins Hospital (detailed clinicopathologic information not available) and mCRPC (n = 56) from the University of Washington rapid autopsy program (2012–2018), were retrieved. Three representative cores were arrayed from each tumor. Specimens and pathologic information were collected with approval from institutional review boards at both institutions. Using the histologic subclassification described earlier, the entire TMA cohort consisted of 43 adenocarcinomas, 15 SmCC, and 12 anaplastic carcinomas. Tumors with all 3 cores on the sections were evaluated.
Immunohistochemistry
TMA tissue sections (5 µm) were immunohistochemically stained for PSMA and FAP. The CASCADE cohort was stained only for FAP because of the unavailability of additional sections. Deparaffinization and antigen retrieval were performed simultaneously using EnVision Flex Target Retrieval Solution (pH 9.0; Agilent Technologies) at 99°C for 20 min in Dako PT Link (Agilent). The slides were then processed on Agilent AutoStainer Link 48 with tailored staining protocols. Background reduction was achieved through a combination of EnVision Flex peroxidase block (catalog number K8002; Agilent) and casein blocking (catalog number SP-5020; Vector Laboratories). Sections were stained using monoclonal mouse anti-PSMA (clone 3E6, 1:400; Agilent) and monoclonal rabbit anti-FAP (clone EPR20021, 1:200; Abcam). Visualization used EnVision Flex horseradish peroxidase–labeled polymer (catalog number SM802; Agilent) and EnVision Flex diaminobenzidine-positive substrate chromogen (catalog number SM803; Agilent). This was followed by counterstaining with EnVision Flex hematoxylin (catalog number SM803; Agilent). Staining intensity and percentage were evaluated manually, independently, and double-masked by 2 pathologists. For both PSMA and FAP, the immunohistochemistry score equaled the intensity score (0–3) multiplied by the percentage score (0%–100%) multiplied by 100, with a 0–300 range. For FAP, the percentage score was defined by the percentage of tumor tissue area containing positive stromal cells. Stained sections were scanned via Aperio ScanScope AT, using the ×20 objective scan setting. Tissue Studio software (Definiens AG) was used to identify, quantify, and interpret immunostains and cellular morphology in tissue samples. For TMA cases, the immunohistochemistry score used for statistical analysis was the pooled mean from 3 cores sampled from each tumor. A binary cutoff for immunopositivity was arbitrarily defined as an overall immunohistochemistry score of at least 10 (intensity score ≥ 2 and percentage score ≥ 5%).
PET/CT Imaging
Preliminary data analysis of 4 patients with metastatic PCa enrolled in the NCT04457232 trial (UCLA institutional review board 20-000177) between October 28, 2020, and December 7, 2021, was performed. This exploratory prospective study aimed to evaluate the biodistribution of [68Ga]FAPi-46 in patients with PCa and whether [68Ga]FAPi-46 and [68Ga]PSMA-11 PET signals correlate with the amount of FAP and PSMA, respectively, in excised cancer tissue. Patients who were scheduled to undergo surgical resection or biopsy of a primary, recurrent, or metastatic PCa lesion were eligible. Patients who received therapy between the PSMA PET/CT scan and the FAPi PET/CT scan were excluded. Patients underwent both [68Ga]FAPi-46 (median injected activity, 196.1 MBq [5.3 mCi]; median acquisition start, 59 min after injection) and [68Ga]PSMA-11 (median injected activity, 177.6 MBq [4.8 mCi]; median acquisition start, 64 min after injection) PET scans within 3 mo (median, 4 d; range, 1–38 d) and underwent a subsequent biopsy in a metastatic lesion. Biopsy tissue sections were used to explore immunohistochemistry and imaging correlation. One PET/CT reader collected the SUVmax and the CT size of the biopsied lesion.
Statistical Analysis
Statistical analyses were performed using GraphPad Prism 9 (GraphPad Software). The Spearman ρ correlation test was used to assess the correlation between manual scores and Definiens imaging analysis scores of FAP. The Wilcoxon rank-sum test was used to compare immunohistochemistry scores between PSMA and FAP on the same tumor samples. The Mann–Whitney U test was used to assess immunohistochemistry results on different histologic subtypes or tissue sites. The Pearson χ2 test and Fisher exact test were used to assess categoric data. A P value of less than 0.05 was considered statistically significant. Figures were generated by GraphPad Prism 9.
RESULTS
Concordant Manual and Automated Assessment of Immunohistochemistry Scores
FAP expression was limited to stromal cells in 114 of 116 cases; in 2 cases, staining was also focally present in tumor cells. Consequently, only stromal cell positivity was recorded. First, we evaluated the degree of concordance between manual scoring and Definiens digital quantification methods on 24 CASCADE samples with variable FAP immunopositivity. The 2 scoring methods demonstrated a statistically significant, strong positive correlation (r = 0.872, P < 0.001), as shown in Supplemental Figure 1 (supplemental materials are available at http://jnm.snmjournals.org). A manual immunoscoring method was adopted for the remaining study because of the cost and efficiency.
Expression Level of FAP in CRPC Is Generally Lower Than That of PSMA
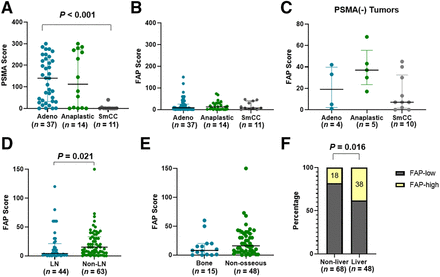
Expression levels of FAP were evaluated using immunohistochemistry in CRPC cases in the TMA cohort (total n = 70) and compared with levels of PSMA, which is an approved theranostic marker for PCa. Representative images of PSMA and FAP immunohistochemistry, scored at 25th, 50th, and 75th percentiles, are shown in Figure 1A. Among the 62 cases for which all 3 cores were present for evaluation, FAP immunoscores (median, 14; interquartile range [IQR], 5–31) were significantly lower than PSMA scores (median, 72; IQR, 1–223; P < 0.001; Fig. 1B), indicating FAPi PET may be inferior to PSMA PET in detecting CRPC lesions in general. Similarly, the transcript levels of FAP were markedly lower than those of FOLH1 (PSMA) in the matched samples from the University of Washington rapid autopsy TMA program (Supplemental Fig. 2) (13).
FAP is expressed at low level in CRPC and independent of PSMA expression. (A) Representative images of PSMA and FAP immunohistochemistry of CRPC samples, with immunoscores at 25th, 50th, and 75th percentile in TMA cohort (×400 magnification). (B) Expression of PSMA and FAP (median with IQR) in TMA cohort. (C) Fractions of FAP-positive cases in PSMA-negative and PSMA-positive CRPC. (D) FAP expression (median with IQR) in PSMA-negative and PSMA-positive CRPC.
FAP Expression in CRPC Is Independent of PSMA Positivity
We arbitrarily defined tumor marker positivity as an immunohistochemistry score of at least 10. Among 62 tumors in the TMA cohort, 19 (31%) were PSMA-negative and 43 (69%) were PSMA-positive. In contrast, 26 (42%) tumors were FAP-negative and 36 (58%) tumors were FAP-positive. PSMA and FAP positivity showed no significant correlation (P = 0.986). The same fractions of tumors were FAP-positive in both the PSMA-negative group (11/19, 58%) and the PSMA-positive group (25/43, 58%; Fig. 1C). The FAP immunohistochemistry scores exhibited no significant difference between the PSMA-negative tumors (n = 19; median, 17; IQR, 5–40) and the PSMA-positive tumors (n = 43; median, 13; IQR, 5–30; P = 0.691; Fig. 1D).
FAP Expression in CRPC Is Differentiation-Independent and Site-Dependent
We assessed PSMA expression in different histologic subtypes of CRPC, including adenocarcinoma, anaplastic carcinoma, and SmCC. SmCC (n = 11) exhibited a significantly lower PSMA score (median, 1; IQR, 0–1) than that of adenocarcinoma (n = 37; median, 140; IQR, 32–225; P < 0.001; Fig. 2A). Anaplastic carcinoma (n = 14) showed levels of PSMA expression (median, 113; IQR, 2–273) that were not significantly different from those of adenocarcinoma (n = 37; median, 140; IQR, 32–225; P = 0.954).
FAP expression in CRPC is differentiation-independent and site-dependent. (A and B) Expression of PSMA (A) and FAP (B; median with IQR) in different histologic subtypes of CRPC in TMA cohort. (C) FAP expression (median with IQR) in different histologic subtypes of CRPC that were PSMA-negative. (D) FAP expression in mCRPC in lymph node and nonnodal locations. (E) FAP expression in mCRPC in bone and nonosseous locations. (F) Fractions of mCRPC with high FAP expression levels in liver and nonliver locations. Adeno = adenocarcinoma; LN = lymph node.
To survey FAP expression across different histologic subtypes and metastatic locations, we combined 2 cohorts and summarized the results in Table 1. In contrast to PSMA, FAP demonstrated an expression level independent of histologic classifications (P = 0.818 between SmCC and adenocarcinoma and P = 0.857 between anaplastic carcinoma and adenocarcinoma; Fig. 2B). The histology subtype independency was also observed in the PSMA-negative group (Fig. 2C).
Correlation of FAP Expression with Clinicopathologic Factors
Next, we evaluated FAP scores in tumors from different tissue locations. The FAP level was significantly lower in lymph node metastasis (n = 44; median, 4; IQR, 1–21) than in distant metastasis (n = 63; median, 15; IQR, 5–48; P = 0.021; Fig. 2D; Table 1). Among nonnodal mCRPC, bone metastases exhibited a trend of lower FAP expression (n = 15; median, 8; IQR, 1–20) than that of nonosseous metastases (n = 48; median, 16; IQR, 5–37), although the difference did not reach statistical significance (P = 0.107; Fig. 2E).
In addition, we examined characteristics of tumors with the highest FAP expression. We dichotomized FAP expression as high FAP versus low FAP at the 75th percentile (immunohistochemistry score, 30). We observed significant enrichment of FAP-high tumors in liver metastases: 38% (18/48) of liver metastases had high FAP, significantly higher than the 18% (12/68) of nonliver samples (P = 0.016; Fig. 2F). There was no significant difference in the distribution of FAP-high tumors between nodal and nonnodal locations.
Correlation of PET Scan Signal and Biopsy Immunohistochemistry in Patients
We conducted a preliminary analysis of 4 patients included in the NCT04457232 trial between October 28, 2020, and December 7, 2021. The patients’ clinical information is provided in Table 2.
Patient Characteristics in Pilot Imaging Immunohistochemistry Correlation Study
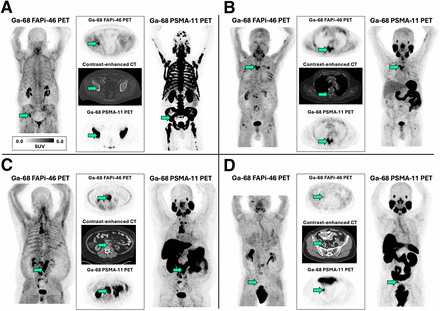
One patient had metastatic castration-sensitive PCa, and 3 patients had mCRPC. The metastatic lesion sites of the biopsy samples were bone (n = 2) and retroperitoneal lymph nodes (n = 2; Fig. 3). The SUV and immunohistochemistry scores of these lesions are provided in Table 3. Similar to the tissue survey results, FAP immunoscores in sampled tumors (median, 30; IQR, 7–40) were markedly lower than PSMA immunoscores (median, 160; IQR, 125–248). On PET imaging, lesion sites of biopsy samples showed lower uptake intensity on FAPi PET (median SUVmax, 9.6; IQR, 4.4–13.4) than on PSMA PET (median SUVmax, 14.5; IQR, 9.3–27.0). The preliminary results suggest lower sensitivity of FAPi PET than of PSMA PET in patients with advanced PCa.
(A–D) Correlation of FAPi and PSMA PET imaging in 4 patients with metastatic PCa. Median imaging interval was 4 d (range, 1–38 d). For each patient, left and right panels show PET maximum intensity projections, and middle panels show transversal planes of PET and CT. Arrows point to lesion that was subsequently biopsied and used for immunohistochemistry analysis.
PET and Immunohistochemistry Scores in Pilot Imaging Immunohistochemistry Correlation Study
DISCUSSION
Tissue expression of FAP in PCa has been evaluated in several recent studies. Two studies reported that FAP expression was minimal to absent from primary PCa, with elevated expression in advanced PCa (19,20). These findings suggest that FAPi PET may have limited utility in patients with localized PCa but could be a potential imaging target for those with metastatic PCa. However, the use of FAPi PET as a standard imaging tool in CRPC patients remains debatable. In this study, we observed that FAP expression levels in CRPC samples were generally lower than those of PSMA (Fig. 1; Supplemental Fig. 2). To our knowledge, the contrasting difference in the expression levels of PSMA and FAP in CRPC samples has not been previously reported. This finding implies that FAPi PET may be less sensitive than PSMA PET in detecting mCRPC lesions in unselected CRPC patients. This conclusion is supported by the findings in our pilot imaging immunohistochemistry correlation study.
Finding low FAP expression in most late-stage PCa is not surprising. Unlike stromogenic cancer types, such as pancreatic adenocarcinoma and cholangiocarcinoma, primary PCa typically lacks a desmoplastic reaction in the stroma. In the normal prostate, prostatic luminal epithelial cells communicate with stromal cells to maintain smooth muscle differentiation (25). Studies indicate significant variability in reactive stroma levels in primary PCa, with most cases showing minimal or no reactive stroma (26,27). Grade group 5 tumors often lack reactive stroma entirely. Only a small percentage (6.7%) of primary PCa was classified as the stromogenic subtype in a biopsy cohort (27). Both stromogenic subtypes and stroma-deficient tumors were associated with increased biochemical recurrence rates. The tumor progression from castration-sensitive PCa to CRPC is associated with elevated FAP expression (19,20), yet a study of 208 mCRPC samples found that only 10% displayed high FAP messenger RNA expression (28). Although FAP is expressed in both stroma and tumor cells in hepatocellular carcinoma, cholangiocarcinoma, and pancreatic adenocarcinoma, its expression in PCa cells is rare (29). Consistent with this, our observations revealed exclusive FAP expression in the stroma, with only 2 of 116 tumors exhibiting focal FAP staining in tumor cells. FAP expression is positively regulated by transforming growth factor-β at the transcription level (29). The precise biologic reasons for the lower FAP expression in mCRPC than in other solid tumors remain unclear.
Because CRPC may lose PSMA expression during disease progression under treatment pressure, we focused on FAP expression in PSMA-negative CRPC. Whether FAP is homogeneously expressed in PSMA-negative CRPC was unknown before our study. As shown in Figures 1D and 2, FAP expression was independent of PSMA expression level and histologic subtypes, indicating that CRPC tends to sustain its ability for stromal modification during late-stage dedifferentiation. FAP expression was heterogeneous in PSMA-negative lesions, including SmCC (Fig. 2C). On the basis of these findings, we hypothesize that FAPi may serve as an alternative or combinational imaging modality in late-stage CRPC patients when their tumors lose PSMA expression. However, FAPi is not likely to detect all PSMA-negative CRPC lesions. In SmCC, which is essentially PSMA-negative, FAPi PET may serve as a complementary tool. Treatment directed to FAP in those specific cases of cancers that are PSMA-negative but FAP-positive remains a concept that would be worth further clinical investigation.
Hintz et al. (20) measured FAP messenger RNA levels in a cohort of mCRPC samples and revealed no significant difference in FAP expression among tumor genotypes, treatment regimens, or locations of metastasis. Our data, measured at the protein level, are mostly in line with their findings. For example, there was no difference in FAP expression among 3 histologic subtypes and no significant difference between bone metastasis and visceral metastasis. However, contrary to their results, we noticed a significantly lower level of FAP expression in lymph node metastases than in nonnodal metastases. Therefore, we hypothesize that an FAPi scan may be less sensitive in detecting nodal lesions than in detecting visceral lesions. In addition, FAP-high tumors were enriched in liver metastases, suggesting that liver metastases may be sensitive to FAPi imaging and FAP-targeted radiopharmaceutical therapy. Our finding on tissue survey needs validation, and the upcoming comprehensive analysis of our prospective FAPi imaging clinical trial might help to yield more robust data.
Results from our preliminary imaging–immunohistochemistry correlation analysis support our proposed cutoff of a FAP immunohistochemistry score of at least 10 to define FAP-positive CRPC, for which FAPi may be able to detect metastatic PCa. Although this cutoff requires further investigation, an imaging-validated immunohistochemistry cutoff would be valuable when selecting patients for future radiopharmaceutical therapy trials.
Our study has some limitations. Cohorts of CRPC tumor samples are difficult to assemble in any single institution because of their rarity. Our tissue samples were a combination of TMA and regular sections from different institutions and resources. Institution-specific preanalytic variables, which we cannot exclude, may affect the immunohistochemistry results. Intratumoral heterogeneity and spatial distribution of FAP-positive stroma were not analyzed. In 4 trial cases, FAPi PET did not detect PSMA PET-negative lesions. Statistical analysis was not performed because of the low n in our pilot imaging–immunohistochemistry correlation in 4 trial patients. This study aimed to generate preliminary data and a hypothesis for the comprehensive correlation study of our FAPi imaging trial.
CONCLUSION
The expression level of FAP in CRPC is generally low, suggesting limited value for FAPi PET imaging in patients with CRPC. The loss of PSMA expression in CRPC does not affect the expression of FAP. Although the utility of FAPi PET in detecting PSMA-negative CRPC could be further investigated, other modern imaging approaches should be evaluated as alternative choices.
DISCLOSURE
Huihui Ye is funded by the UCLA Jonsson Comprehensive Cancer Center Fund and UCLA Prostate SPORE. Jeremie Calais received a grant from the Prostate Cancer Foundation for the clinical portion of this work (2020 PCF Young Investigator Award 20YOUN05). Adrien Holzgreve is funded by the Deutsche Forschungsgemeinschaft (545058105). The UW rapid autopsy specimens are the result of work supported by resources from the Department of Defense Prostate Cancer Research Program (W81XWH-14-2-0183), the Pacific Northwest Prostate Cancer SPORE (P50CA97186), and the Institute for Prostate Cancer Research. Peter Nelson is supported by P50CA097186, R01CA266452, and R01CA234715. Johannes Czernin is a founder of SOFIE Biosciences and holds equity in the company and in intellectual property invented by him, patented by the University of California, and licensed to SOFIE Biosciences. He is a founder and board member of Trethera Therapeutics and holds equity in the company and in intellectual property invented by him, patented by the University of California, and licensed to Triangle. He serves on the medical advisory board of Actinium Pharmaceuticals and on the scientific advisory boards of POINT Biopharma, RayzeBio, and Aktis Oncology. No other potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: Can FAPi PET detect CPRC lesions that are missed by PSMA PET?
PERTINENT FINDINGS: In a retrospective tissue survey, PSMA-negative CRPC lesions were found to express FAP at levels similar to those of PSMA-positive CRPC. FAP expression levels in CRPC are generally lower than those of PSMA, a finding supported by preliminary results from an imaging trial.
IMPLICATIONS FOR PATIENT CARE: Although FAPi PET is likely less sensitive than PSMA PET in detecting CRPC lesions, it may have theranostic value in a subset of patients who develop PSMA-negative CRPC.
ACKNOWLEDGMENT
We thank Dr. Tamara Lotan, Johns Hopkins Hospital Department of Pathology, for providing the TMA of locally advanced CRPC for this study.
Footnotes
Guest Editor: Todd Peterson, Vanderbilt University
Published online Oct. 30, 2024.
- © 2024 by the Society of Nuclear Medicine and Molecular Imaging.
REFERENCES
- Received for publication May 8, 2024.
- Accepted for publication September 25, 2024.