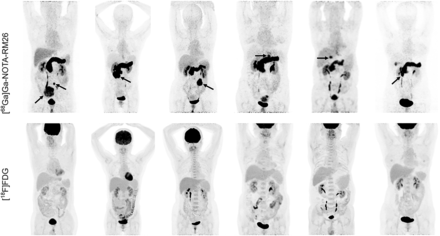
Visual Abstract
Abstract
Gastrointestinal stromal tumors (GISTs) are the most common stromal tumors in the gastrointestinal tract. This study was designed to evaluate a gastrin-releasing peptide receptor antagonist PET tracer, [68Ga]Ga-NOTA-RM26, and compare it with [18F]FDG PET/CT in the assessment of patients with GISTs. Methods: With institutional review board approval and informed consent, 30 patients with suspected or proven GISTs based on abdominal CT or gastroscopy were recruited. All patients underwent [68Ga]Ga-NOTA-RM26 and [18F]FDG PET/CT scans. Pathology and other patient information were collected. Results: No radiopharmaceutical-related adverse events were observed in the patients. In total, 18 lesions in 16 patients were diagnosed as GIST, 3 patients were diagnosed with schwannoma, and 4 patients were diagnosed with leiomyoma. In 18 GISTs, the mean SUVmax of [68Ga]Ga-NOTA-RM26 PET was significantly higher than that of [18F]FDG PET (17.07 ± 19.57 vs. 2.28 ± 1.65; P < 0.01), and [68Ga]Ga-NOTA-RM26 PET/CT had a higher tumor detection rate than did [18F]FDG PET/CT (88.9% vs. 50%; P < 0.01). The uptake of [68Ga]Ga-NOTA-RM26 in GISTs was significantly higher than that in 2 other benign tumors (leiomyoma or schwannoma) (17.07 ± 19.57 vs. 4.23 ± 1.77; P = 0.014). With the SUVmax cutoff value of 6.0, the sensitivity of 68Ga-NOTA-RM26 PET/CT in diagnosing GISTs is 72% and the specificity is 85.7%. Conclusion: Compared with [18F]FDG PET/CT, [68Ga]Ga-NOTA-RM26 PET/CT is a promising and effective imaging modality for the detection of GISTs.
- gastrin-releasing peptide receptor
- [68Ga]Ga-NOTA-RM26
- gastrointestinal stromal tumor
- GRPR antagonist
- [18F]FDG
Gastrointestinal stromal tumors (GISTs) are the most common stromal tumors in the gastrointestinal tract, accounting for less than 1% of gastrointestinal tumors, with the stomach and small intestine being the most common tumor origin sites. GISTs are thought to arise from the precursor of Cajal interstitial cells, typically found in the muscular plexus. Approximately 95% of GIST patients have KIT receptor tyrosine kinase (CD117) mutations (1), whereas most remaining patients have platelet-derived growth factor receptor α mutations, distinguishing them clearly from other mesenchymal tumors, such as leiomyomas, leiomyosarcomas, or neurogenic tumors (2). Most GISTs are initially diagnosed as localized disease, but during disease follow-up, many patients suffer recurrence and metastasis. For GISTs with a high risk of metastasis, high-sensitivity and noninvasive detection methods will allow early detection of lesions for further appropriate treatment. With the increasing understanding of GISTs, imaging has become important not only for diagnosing GISTs but also for monitoring treatment response and detecting tumor progression.
[18F]FDG PET/CT has been reported as a useful imaging modality that complements CT and MRI for staging GISTs and monitoring response to adjuvant imatinib therapy. The National Comprehensive Cancer Network task force report recommends the use of [18F]FDG PET/CT for further evaluation of progressive GISTs after imatinib treatment (3). Although [18F]FDG is the most widely used PET tracer, it has many limitations. Additionally, the role of [18F]FDG PET/CT in GIST patients should be further explored, given that the limited studies available have yielded inconsistent findings. For the early detection of GISTs, studies using [18F]FDG PET/CT have reported low to moderate sensitivity; [18F]FDG PET/CT may not be specific and sensitive enough for small tumors, especially for gastric and peritoneal lesions smaller than 5 mm (4). Therefore, more accurate, specific, and sensitive noninvasive diagnostic tools are needed to detect GISTs. It has been observed that bombesin (BBN) subtype 2 receptor is overexpressed in most GIST cases by in vitro receptor autoradiography (5). Gastrin-releasing peptide receptor (GRPR)–associated binding peptides have shown significant specificity in binding to all GIST cell lines in vitro, and incorporation of radiotracers does not alter the internalization behavior of peptide conjugates (6). GRPR-targeted molecular imaging has been widely investigated in nuclear oncology, and ongoing research focuses on optimizing different radiotracers (7–14). Recently, a new radiolabeled GRPR antagonist, [68Ga]Ga-NOTA-RM26 ([68Ga]Ga-1,4,7-triazacyclononane-N,N′,N′′-triacetic acid-d-Phe-Gln-Trp-Ala-Val-Gly-His-Sta-Leu-NH2, abbreviated as [68Ga]Ga-RM26), was developed with better tumor affinity and fewer side effects than the agonist BBN (15). Our studies have previously reported on the evaluation of [68Ga]Ga-NOTA-BBN and [68Ga]Ga-RM26 in prostate, glioma, and breast cancer patients and found that they have diagnostic value in these patients (7,16–18), and the higher tumor-to-background ratio allowed for the detection of more lesions. Considering that [18F]FDG uptake is insufficient for PET/CT detection of small GISTs, the development of complementary tracers, such as [68Ga]Ga-RM26, is important for more accurate monitoring of GIST patients.
In this study, we intend to use [68Ga]Ga-RM26 PET/CT in a head-to-head comparison with [18F]FDG PET/CT to compare their performance in primary and recurrent metastatic GIST detection.
MATERIALS AND METHODS
Subject Enrollment
This study was registered on clinicaltrials.gov (NCT05001204). The inclusion and exclusion criteria of the study can be found in the supplemental materials (supplemental materials are available at http://jnm.snmjournals.org). With written informed consent, 30 participants suspected or proven of having primary or recurrent GISTs based on CT or gastroscopy were recruited at Peking Union Medical College Hospital from May 2021 to December 2021. The patients’ age, sex, lesion location, diameter, duration of disease, history of treatment (tyrosine kinase inhibitors, for example, imatinib, sunitinib), pathologic type, and vital signs after administration were recorded.
Radiopharmaceutical Preparation
The NOTA-RM26 precursor was purchased from TZ-Bio (Tanzhenbio). Radiolabeling of [68Ga]Ga-RM26 was performed in the sterile hot cell as previously reported (7). Detailed information about labeling is available in the supplemental materials. Only [68Ga]Ga-RM26 with a radiochemical purity greater than 95%, measured by instant thin-layer chromatography, was approved for injection. [18F]FDG was produced by a good manufacturing practice–certified cyclotron for clinical use.
PET/CT Examination Procedures
All participants underwent [68Ga]Ga-RM26 PET/CT scans using a time-of-flight PET/CT scanner (PoleStar m660, SinoUnion) 51 ± 11 min after intravenous injection of approximately 87 ± 31.5 MBq (2.35 ± 0.85 mCi) of [68Ga]Ga-RM26 within 1 wk before the scheduled surgery. CT images were acquired using a low-dose CT scan for localization, and a PET scan was acquired of each patient in 5–6 bed positions (2 min/bed) from the upper thigh to the skull base. [18F]FDG PET/CT was performed after the routine procedure, with approximately 5.55 MBq/kg (0.15 mCi/kg) of [18F]FDG.
Image and Data Analysis
The PET/CT images were evaluated visually in the axial, sagittal, and coronal planes. Two experienced nuclear medicine physicians independently evaluated all PET images by opening 2 PET scans of the same patient at the same workstation. The uptake of lesions on the PET scan was semiquantitatively assessed using the SUVmax. Any focal uptake of [68Ga]Ga-RM26 and [18F]FDG that was higher than the surrounding background activity and could not be explained by physiologic uptake was considered positive. The background SUVmax was obtained from surrounding normal organs (i.e., the stomach and intestine). The 2 physicians were masked to the location of the GIST before obtaining the PET image data and were unaware of any other imaging data, pathologic results of biopsy or surgery, or the medication status of the patients (Supplemental Table 1). Any disagreements were resolved by discussion until a consensus was reached.
Immunohistochemical Staining of GRPR Expression
We examined the immunohistochemical features of the obtainable specimens in 17 of 26 cases. GRPR antibody (ThermoFisher, PA5-26791, antirabbit) was used for staining at a dilution of 1:100. The immunoreactive score of GRPR staining (in the range of 0 to 12) was quantified by an experienced pathologist in accordance with staining intensity and the percentage of positive tumor cells according to published recommendations (17).
Statistical Analysis
All statistical analyses were performed using Prism 9.0 (GraphPad). All continuous data were expressed as mean ± SD. Noncontinuous data were presented as counts or percentages. The McNemar test was used to assess the difference in lesion detection rates for GISTs between [68Ga]Ga-RM26 and [18F]FDG PET/CT. The Wilcoxon signed-rank test was used to compare the SUVs and the tumor-to-background ratios of primary and metastatic GISTs detected by [68Ga]Ga-RM26 and [18F]FDG PET/CT. The receiver operating characteristic curve analysis was performed to determine an optimal cutoff value for detecting GISTs from 2 other benign tumors (leiomyoma or schwannoma) and to determine the diagnostic performance. The Spearman correlation coefficient was used to measure the correlation between the SUVs and the immunohistochemical staining score. A 2-tailed P value of less than 0.05 indicated statistical significance.
RESULTS
Adverse Events
No radiopharmaceutical-related adverse events were observed in the patients. A simple case report form that records the patients’ basic vital signs and adverse events within 24 h after administration is provided in Supplemental Table 2.
Patient Characteristics
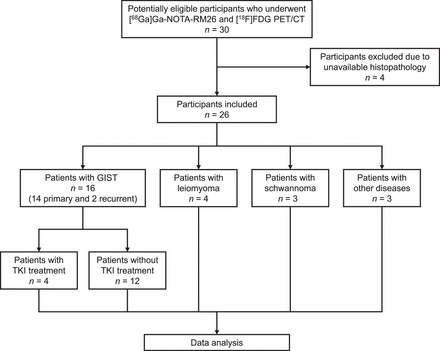
A total of 30 participants underwent [68Ga]Ga-RM26 PET/CT examinations; 4 participants were excluded from the data analysis because of missing or uncertain pathologic results. The final group in this study consisted of 26 participants, including 16 patients with GISTs, 4 patients with leiomyomas, 3 patients with schwannomas, 2 patients with heterotopic pancreata, and 1 patient with endometriosis (Fig. 1). The mean age of the participants was 56 ± 12 y (range, 21–71 y). In total, 21 participants were women (70%) and 9 participants were men (30%). The mean diameter of all lesions was 4.0 ± 2.4 cm. Among the 16 patients with primary or recurrent GISTs, the mean time to first presentation was 15.4 ± 16.3 mo. Among the 18 GISTs in 16 patients, 10 lesions (55.6%) were located in the stomach, 4 lesions (22.2%) in the small intestine, 2 lesions (11.1%) in the peritoneal cavity, 1 lesion (5.6%) in the mesentery, and 1 lesion (5.6%) in the peritoneum. Four GIST patients had a history of prior treatment with tyrosine kinase inhibitors, of which 3 received first-line treatment (imatinib) and 1 received second-line treatment (sunitinib). Further details on demographics and disease status are summarized in Table 1.
Flow diagram. TKI = tyrosine kinase inhibitor.
Patient Characteristics
[68Ga]Ga-RM26 and [18F]FDG PET/CT Per-Lesion Analysis in GIST Patients
When all 18 pathologically confirmed GISTs were compared 1 by 1 in [68Ga]Ga-RM26 and [18F]FDG PET/CT, [68Ga]Ga-RM26 PET/CT showed a good diagnostic performance in patients with GISTs (Figs. 2 and 3). [68Ga]Ga-RM26 PET/CT successfully diagnosed 16 of all 18 GISTs (88.9%), outperforming [18F]FDG PET/CT, which diagnosed only 9 of 18 GISTs (50%). This difference was statistically significant (P < 0.05). The mean SUVmax of [68Ga]Ga-RM26 in GISTs was 17.07 ± 19.57, significantly higher than the SUVmax of [18F]FDG, which was only 2.28 ± 1.65 (P < 0.01).
Representative images of comparison CT, [18F]FDG PET/CT, and [68Ga]Ga-RM26 PET/CT in patients 16 (A), 13 (B), 15 (C), and 10 (D), with GISTs in different regions.
Performance of [68Ga]Ga-RM26 and [18F]FDG PET/CT in patient 2 with recurrent GIST and 2 lesions. [68Ga]Ga-RM26 PET/CT scan showed intense uptake in both lesions (SUVmax, 12.15 and 14.21), and there was no enhancement of mesenteric lesion (smaller one) on contrast-enhanced (ce) CT (arterial phase) and no FDG avidity of lesion on [18F]FDG PET/CT.
[68Ga]Ga-RM26 PET/CT in Primary Benign Gastrointestinal Tumor Subtypes
Leiomyomas and schwannomas are 2 common benign tumors originating from the gastrointestinal tract. In our pilot study, 4 patients with leiomyomas and 3 patients with schwannomas were included, and these participants also underwent [68Ga]Ga-RM26 and [18F]FDG PET/CT scans for comparison. The uptake of [68Ga]Ga-RM26 in leiomyomas was slightly higher than that of [18F]FDG, with mean SUVmax values of 5.15 ± 1.69 and 2.41 ± 1.10, respectively. The uptake of [68Ga]Ga-RM26 in schwannoma was lower and the uptake of the [18F]FDG tracer was relatively high, with mean SUVmax of 3.02 ± 1.13 and 5.12 ± 0.80, respectively. It is worth noting that [18F]FDG could not distinguish GISTs from the other 2 benign tumors (mean SUVmax, 2.28 ± 1.65 for GIST vs. 2.41 ± 1.2 for the benign tumors; P > 0.05), whereas [68Ga]Ga-RM26 could well distinguish GISTs from the other 2 tumors (mean SUVmax, 17.07 ± 19.57 vs. 4.23 ± 1.77; P = 0.014) (Fig. 4). To evaluate the diagnostic ability of 68Ga-RM26 for GIST detection, we calculated the receiver operating characteristic curve analysis (Supplemental Fig. 1A; Supplemental Table 3). The results showed that when the cutoff value of SUVmax is defined as 6.0, the sensitivity of 68Ga-RM26 PET/CT in diagnosing GIST is 72% and the specificity is 85.7%. The area under the curve is 0.818 (P = 0.016).
Representative [68Ga]Ga-RM26 and [18F]FDG PET/CT images and immunohistochemically stained tissue samples from leiomyoma and schwannoma patients. (A) A 26-y-old woman (patient 12) with schwannoma on gastric corpus. (B) Schwannoma sample of patient 15 showed nearly no GRPR overexpression (×20). (C) A 65-y-old woman (patient 1) with leiomyoma on gastric cardia. (D) Leiomyoma sample of patient 1 was mildly positive for GRPR (×20).
[68Ga]Ga-RM26 PET/CT and Overexpression of GRPR
In total, 18 histopathologic samples from surgery or endoscopic biopsy were available for GRPR expression analysis. We performed GRPR immunohistochemical staining on these pathologic samples, and these results were compared with SUVmax of the corresponding lesions on [68Ga]Ga-RM26 PET/CT to demonstrate the correlations. The SUVmax and immunoreactive scores of the lesions can be found in Table 1. There was a significant positive correlation between SUVmax from [68Ga]Ga-RM26 PET/CT and GRPR expression with an immunoreactive score (Spearman correlation analysis, r = 0.8532, P < 0.0001) (Supplemental Fig. 1B).
DISCUSSION
In a previous study, a [68Ga]Ga-labeled BBN analog, [68Ga]Ga-BZH3, showed lower uptake than [18F]FDG and detected fewer lesions than [18F]FDG (19). This stands in direct contrast to our results, which suggest that [68Ga]Ga-RM26 imaging may be a promising tool for detecting new lesions or metastases compared with conventional [18F]FDG PET/CT or [68Ga]Ga-labeled BBN. In our pilot study, patients 2 and 17, with recurrence and metastases, were both found to have an additional previously unknown lesion on [68Ga]Ga-RM26 PET/CT. The high tumor uptake, high tumor-to-kidney ratio, and relatively long tumor residence time of RM26 suggest its potential not only for use in imaging but also for use in radiopharmaceutical therapy in primary and metastatic tumors with high GRPR expression.
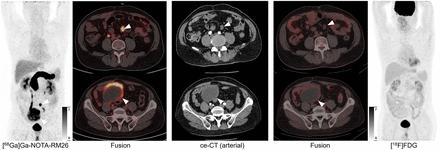
The staging effect of [18F]FDG PET/CT in GIST patients has been confirmed by some studies, and the correlation between [18F]FDG uptake and the malignant potential or prognosis of GIST has also been demonstrated (20–24). Several studies have described the role of [18F]FDG PET/CT in monitoring the response to treatment in GIST patients, including early response to imatinib (1,24–26). The change in [18F]FDG uptake before and after treatment is considered an early predictor for patients receiving imatinib as a first-line advanced treatment or sunitinib as a second-line treatment. However, glucose metabolism may not fully reflect the proliferation of tumor cells in GISTs, and abundant extracellular components in GISTs and inflammation due to hemorrhage and necrosis may also alter [18F]FDG uptake. Therefore, [18F]FDG uptake may be partially, but not completely, related to tumor cell proliferation. We believe that this may be related to the limitations of uptake intensity in predicting proliferation and invasiveness. The false-negative results of [18F]FDG may be related to tumor size (likely due to resolution and the partial-volume effect) and also to low glucose metabolism of tumor cells with low mitotic index and low Ki-67 score. Figure 5 shows a notable case of a 45-y-old female patient (patient 11), who recently presented to the hospital with abdominal discomfort. Enhanced CT showed a lobulated hypervascular tumor with significant contrast enhancement in the right lower abdomen, with 2 nonenhancing necrotic areas. On [18F]FDG PET/CT, the solid part of the tumor had no FDG avidity and the necrotic area had relatively high FDG avidity (SUVmax, 2.66). On [68Ga]Ga-RM26 PET/CT, we found that the tumor had an intense uptake (SUVmax, 79), and the nonenhancing areas on enhanced CT in the arterial phase had a relatively low uptake of [68Ga]Ga-RM26. The patient underwent surgery and was diagnosed with GIST (tumor length, 6.3 cm; Ki-67, 5%; mitotic index, 0–2; assayed with low grade). This case strongly suggests that necrosis may occur with neutrophil or macrophage aggregation, leading to [18F]FDG uptake in the necrotic part, whereas the necrotic part does not express GRPR because of a lack of tumor cells, which suggested that [18F]FDG uptake may not necessarily be a predictive indicator for evaluating the malignancy of GISTs. It is worth noting that the lesions of 2 patients (patients 21 and 22) were assessed as negative, with an SUVmax similar to that of the surrounding gastric wall. The immunoreactive score of the lesion in patient 22 was 3, which was consistent with the uptake value on [68Ga]Ga-RM26 PET/CT (SUVmax, 1.86). As these 2 patients had not received any treatment before the PET examination, it is speculated that this was due to the heterogeneity of the GIST.
Representative patient with GIST. (A) Enhanced CT showed 2 nonenhancing necrotic parts within tumor. On [68Ga]Ga-RM26 PET/CT, lesion had extremely high tracer uptake (SUVmax, 79). On [18F]FDG PET/CT, solid part of tumor had no FDG avidity, and necrotic area had relatively high FDG avidity (SUVmax, 2.66). (B) On [68Ga]Ga-RM26 PET/CT, nonenhancing areas on contrast-enhanced (ce) CT in arterial phase had relatively low uptake of [68Ga]Ga-RM26. Stained GIST sample was positive for GRPR (×40).
GISTs, leiomyomas, and schwannomas are common benign tumors of the gastrointestinal tract. Some previous studies have shown that both GISTs and leiomyomas express GRPR, and GISTs with extremely high densities (GISTs: 16/19, average density, 7,865 ± 9,628 disintegrations per min/mg of tissue; leiomyoma: 20/26, average density, 2,207 ± 623 disintegrations per min/mg of tissue) (5,27). A highlight of our study is that some patients with leiomyomas and schwannomas underwent both [68Ga]Ga-RM26 and [18F]FDG PET/CT imaging, with results suggesting that [68Ga]Ga-RM26 PET/CT but not [18F]FDG PET/CT was able to differentiate GIST from these 2 benign tumors. It is well known that leiomyomas and schwannomas have an extremely low risk of malignant transformation, and many of them are discovered incidentally during CT scans or gastrointestinal endoscopy. In asymptomatic patients with no dysphagia, bloating, abdominal pain, or other discomfort, if PET scans with [68Ga]Ga-RM26 and [18F]FDG show low uptake in the lesion, and particularly if [68Ga]Ga-RM26 uptake is not high, a watch-and-wait approach to follow-up may be adopted to avoid unnecessary surgery and thereby change the management of disease.
Our study has several limitations. First, this is a single-center study, and the number of patients is relatively small. In addition, the patients recruited in this study were mostly those with primary GISTs, and we only included 2 patients with recurrent and metastatic GISTs. Therefore, a study including more patients with nonprimary GISTs is warranted for comparing [68Ga]Ga-RM26 with [18F]FDG in terms of diagnostic efficiency and uptake value in more metastatic lesions, and with MRI in the diagnosis of liver metastasis or peritoneal metastasis. In addition, [18F]FDG PET/CT could be used to predict prognosis in monitoring GIST patients after tyrosine kinase inhibitor treatment. However, the role of [68Ga]Ga-RM26 PET/CT in the management of patients receiving targeted therapy is unclear and requires further investigation.
CONCLUSION
This study evaluated the potential role of [68Ga]Ga-RM26 PET/CT in diagnosing patients with primary and recurrent GISTs. We found that [68Ga]Ga-RM26 outperformed [18F]FDG and detected more GISTs with significantly higher uptake, suggesting its usefulness for tumor localization and staging. These results support [68Ga]Ga-RM26 as a potential tool for the assessment of localized and metastatic gastrointestinal masses. Based on the high expression of GRPR in GISTs, therapeutic radionuclide-labeled RM26 and its analogs should be studied for their potential use in radiopharmaceutical therapy of unresectable or recurrent metastatic GISTs. These preliminary results suggest that further research in a larger cohort of patients is warranted.
DISCLOSURE
This work was financially supported by the National High Level Hospital Clinical Research Funding (2022-PUMCH-D-002, 2022-PUMCH-C-004), Chinese Academy of Medical Science Innovation Fund for Medical Sciences (2021-I2M-1-016, 2022-I2M-2-002, 2022-I2M-C&T-A-008), the National Natural Science Foundation of China (82272046), the National University of Singapore (NUHSRO/2021/097/Startup/13; NUHSRO/2020/133/Startup/08; NUHSRO/2023/008/NUSMed/TCE/LOA), National Medical Research Council (MOH-001334-00, MOH-001388-00, MOH-001254-01, CG21APR1005), the Singapore Ministry of Education (FY2022) Tier 1 Grant (NUHSRO/2022/093/T1/Seed-Sep/06), and the NUS School of Medicine Nanomedicine Translational Research Programme (NUHSRO/2021/034/TRP/09/Nanomedicine). No other potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: Is [68Ga]Ga-RM26 PET/CT superior to [18F]FDG PET/CT in detecting GISTs?
PERTINENT FINDINGS: In this prospective cohort study involving 18 lesions in 16 patients, [68Ga]Ga-RM26 PET/CT exhibited a higher detection rate or increased uptake compared with [18F]FDG PET/CT in GISTs.
IMPLICATIONS FOR PATIENT CARE: [68Ga]Ga-RM26 PET/CT may be a promising tool for the identification of GISTs in the case of negative [18F]FDG findings or suspicion of GISTs.
Footnotes
↵* Contributed equally to this work.
Published online Oct. 24, 2024.
- © 2024 by the Society of Nuclear Medicine and Molecular Imaging.
REFERENCES
- Received for publication March 28, 2024.
- Accepted for publication September 24, 2024.