Visual Abstract
Abstract
[177Lu]Lu-prostate-specific membrane antigen (PSMA) is an effective treatment for metastatic castration-resistant prostate cancer (mCRPC). [177Lu]Lu-PSMA SPECT/CT 24 h after injection has shown potential as a response biomarker for [177Lu]Lu-PSMA therapy but is not convenient for patients. This study investigated 4-h [177Lu]Lu-PSMA SPECT/CT as an alternative to 24-h [177Lu]Lu-PSMA SPECT/CT for evaluation of treatment response. Methods: This prospective analysis enrolled 23 patients diagnosed with mCRPC commencing [177Lu]Lu-PSMA-I&T therapy. Two patients were excluded because of incomplete imaging data. Posttherapy SPECT/CT was performed at 4 and 24 h after the first dose and 4 h after the second dose. Baseline [68Ga]Ga-PSMA-11 PET/CT and 4- and 24-h [177Lu]Lu-PSMA SPECT/CT were analyzed both visually and semiquantitatively. Bland–Altman plots assessed agreement of semiquantitative parameters from the 4- and 24-h scans. Quantitative assessment of the change in the total tumor volume (TTV) on the 4-h [177Lu]Lu-PSMA SPECT/CT after the first and second doses was correlated to patient outcomes. Results: All patients had mCRPC previously treated with an androgen receptor pathway inhibitor, and 11 (52%) received prior taxane chemotherapy. Median age was 78 y, and median prostate-specific antigen level was 54 ng/mL. On visual analysis, disease distribution was unchanged among the 3 imaging methods. Eleven patients (52%) had a median of 1 lesion not identified on 4-h [177Lu]Lu-PSMA SPECT/CT compared with 24-h [177Lu]Lu-PSMA SPECT/CT. All missed lesions on the 4-h [177Lu]Lu SPECT/CT were smaller than 2 cm. Mean differences and agreement between 4- and 24-h SPECT/CT quantitative parameters were within acceptable bounds for lesion number, SUVmax, and SUVmean, with higher variation observed for TTV. The change in TTV between dose 1 and 2 [177Lu]Lu-PSMA SPECT/CT predicted prostate-specific antigen progression-free survival. Conclusion: [177Lu]Lu-PSMA SPECT/CT at 4 h after injection appears a promising alternative to 24-h [177Lu]Lu-PSMA SPECT/CT for treatment response assessment, with improved patient convenience.
In men with metastatic castration-resistant prostate cancer (mCRPC) for whom androgen receptor pathway inhibition and taxane chemotherapy have failed, [177Lu]Lu-prostate-specific membrane antigen (PSMA) is an effective therapy that improves overall survival (1,2). Interim PSMA PET/CT has been evaluated for determining treatment response to [177Lu]Lu-PSMA, effectively predicting both prostate-specific antigen (PSA) progression-free survival and PSA overall survival, but has a high cost and limited availability (3). [177Lu]lutetium can be used for whole-body SPECT, as can other radiopharmaceutical therapies entering the clinic, including 67Cu and 212Pb (4,5). Several trials, including TheraP, have used [177Lu]Lu-PSMA SPECT/CT at 24 h to assess retention in target and off-target tissues (1,6,7). Several studies have confirmed the ability of [177Lu]Lu-PSMA SPECT/CT at 24 and 48 h to predict response to [177Lu]Lu-PSMA treatment as early as of 6 wk (8–11). Acquiring the SPECT/CT images at 4 h after [177Lu]Lu-PSMA injection could represent a more convenient alternative to later time points for patients, allowing scanning on the same day as treatment. This study aimed to evaluate 4-h [177Lu]Lu-PSMA SPECT/CT as an alternative to 24-h [177Lu]Lu-PSMA SPECT/CT for evaluation of treatment response.
MATERIALS AND METHODS
This prospective study included 23 men with mCRPC treated with [177Lu]Lu-PSMA-I&T at St Vincent’s Hospital in an ethics-approved [177Lu]Lu-PSMA-I&T therapy program between May and November 2023. Inclusion criteria included an [68Ga]Ga-PSMA-11 PET/CT scan obtained within 3 mo of commencement of [177Lu]Lu-PSMA-I&T therapy and demonstrating suitable molecular features (SUVmax ≥ 15 and SUVmax ≥ 10 at all measurable lesions not impacted by partial volume [>2 cm]), mCRPC, and Eastern Cooperative Oncology Group status of 2 or less. The Human Research Ethics Committee of St. Vincent’s Hospital approved this study (approval 2022/ETH00924), and all patients gave written consent for the [177Lu]Lu-PSMA-I&T therapy and posttherapy imaging.
Drug Administration
The PSMA-I&T precursor (ABX/Huayi) in sodium acetate buffer was radiolabeled to carrier-free [177Lu]lutetium chloride according to the institutional production protocol. Quality control tests for radionuclide and radiochemical purity were performed using high-pressure liquid chromatography and thin-layer chromatography. Blood was prospectively collected to assess adverse events and biochemical responses.
[177Lu]Lu-PSMA-I&T Therapy
[177Lu]Lu-PSMA-I&T was administered as a slow intravenous bolus (8.5 GBq). Patients were prehydrated orally. Ondansetron, 4 mg, and dexamethasone, 8 mg, were administered orally to patients with high-volume disease. As per institutional and governmental requirements, patients were isolated in a lead-lined room until radiation emission at 1 m was below 25 μSv/h. The 4-h SPECT/CT was undertaken while patients were still in the department, with patients returning for the 24-h scan.
[177Lu]Lu-PSMA SPECT/CT Acquisition
[177Lu]Lu-PSMA SPECT/CT (vertex to mid thighs) was performed for all patients both at 4 h and at 24 h after injection of the first dose of [177Lu]Lu-PSMA-I&T and at 4 h after injection of the second dose. A Discovery NM/CT 870 DR system (GE HealthCare) was used for the acquisition, with medium-energy collimators, 3 bed positions, 60 projections over 360°, an acquisition time of 10 s per frame, a 128 × 128 matrix, and a 4.42 × 4.42 mm pixel size. The energy window was centered on 208 keV ± 10%, with a 165 keV ± 6.5% scatter window. An unenhanced low-dose CT scan was obtained immediately afterward, using the following parameters: pitch of 1, tube voltage of 120 kVp, automatic mAs control (reference mAs of 90), slice thickness of 3.7 mm, matrix of 512 × 512, and field of view of 40 cm. The acquisition time was 25 min at each time point.
SPECT projections were reconstructed with an iterative ordered-subset expectation maximization algorithm using 4 iterations and 10 subsets. No pre- or postreconstruction filters were applied. CT-based attenuation correction, dual-energy-window scatter correction, collimator-based resolution recovery, and quantitative conversion to SUV were performed during the reconstruction on a GE HealthCare SmartConsole. Diagnostic contrast-enhanced CT of the chest, abdomen, and pelvis was undertaken at each treatment cycle to assess for non–PSMA-avid visceral disease progression.
Visual Analysis of [68Ga]Ga-PSMA-11 PET/CT and [177Lu]Lu-PSMA SPECT/CT
Visual analysis of the screening [68Ga]Ga-PSMA-11 PET/CT and the 4- and 24-h [177Lu]Lu-PSMA SPECT/CT after injection of the first dose of [177Lu]Lu-PSMA-I&T was performed by 2 experienced nuclear medicine specialists. Four-hour [177Lu]Lu-PSMA SPECT/CT was compared with 24-h [177Lu]Lu-PSMA SPECT/CT with reference to the screening [68Ga]Ga-PSMA-11 PET/CT to assess disease distribution and determine the number and size of lesions and the types of organs harboring lesions.
Quantitative Analysis of [68Ga]Ga-PSMA-11 PET/CT and [177Lu]Lu-PSMA SPECT/CT
Semiautomated segmentation of screening [68Ga]Ga-PSMA-11 PET/CT and 4- and 24-h [177Lu]Lu-PSMA SPECT/CT after the first and second doses of [177Lu]Lu-PSMA-I&T was performed using LesionID (MIM Software Inc.) and a standardized semiautomated workflow to delineate regions of interest with a minimum SUVmax cutoff of 3 and lesion size of at least 0.5 mm. All lesions identified quantitatively were reviewed by experienced nuclear medicine physicians. Output parameters included SUVmean, SUVmax, and total tumor volume (TTV).
Patient Outcomes
The change in TTV (ΔTTV) was defined by the percentage difference in [177Lu]Lu-PSMA SPECT/CT TTV at 4 h after the first and second doses. This was categorized as a ΔTTV of more than −30% or −30% or less (11). We also assessed whether there was a fall in PSA by 50% or more (PSA-50) from immediately before cycle 1 to any time after treatment commenced. PSA progression was defined as a PSA level at least 20% greater than the nadir or death, with the analysis time beginning on the date of the second dose. Patients who did not have any recorded fall in PSA were defined as experiencing the event after 1 d from the second dose. One patient died before the second dose and was not analyzed for patient outcomes. None of the patients had new PSMA-avid or non–PSMA-avid lesions at 6 wk.
Statistical Analysis
Bland–Altman plots were used to assess agreement between number of lesions, TTV, SUVmax, and SUVmean on the 4- and 24-h [177Lu]Lu-PSMA SPECT/CT. For each variable, standard absolute difference plots were generated, with the arithmetic mean of the 2 variables on the x-axis and horizontal 95% limits of agreement, as well as ratio plots with the geometric mean on the x-axis and regression-derived 95% limits of agreement. Agreement within a ratio of 0.66–1.50 was considered acceptable. Further analysis included calculating the concordance correlation coefficient, with less than 0.90 indicating poor concordance; 0.90–0.95, moderate; 0.95–0.99, substantial; and more than 0.99, almost perfect (12). The association between ΔTTV and PSA progression-free survival was evaluated with Kaplan–Meier curves (log-rank tests). Analyses were performed using Stata version 17.0SE (StataCorp LLC).
RESULTS
Patient Characteristics
Between May and November 2023, 23 patients with mCRPC referred for the [177Lu]Lu-PSMA-I&T therapy program consented to multiple–time-point [177Lu]Lu-PSMA SPECT/CT at St Vincent’s Hospital. [177Lu]Lu-PSMA SPECT/CT was performed 4 h (250 ± 16 min) and 24 h (1,447 ± 55 min) after dose administration. Twenty-one of 23 (91%) men had analyzable screening [68Ga]Ga-PSMA-11 PET/CT scans, and [177Lu]Lu-PSMA SPECT/CT data at 4 and 24 h were included in the analysis; 2 patients were excluded from analysis because of incomplete imaging data. The median age was 78 y (interquartile range [IQR], 70–81 y). All patients had received at least 1 prior androgen receptor pathway inhibitor, 11 (52%) had received docetaxel, and 5 (24%) had received cabazitaxel. All patients received an 8.5-GBq dose of [177Lu]Lu-PSMA-I&T before their SPECT/CT imaging time points. The median time between [68Ga]Ga-PSMA-11 PET/CT for screening and the first dose of [177Lu]Lu-PSMA-I&T was 34 d (IQR, 24–37 d). Patient characteristics are summarized in Table 1.
Patient Characteristics (n = 21)
Visual Comparison of [177Lu]Lu-PSMA SPECT/CT and [68Ga]Ga-PSMA-11 PET/CT
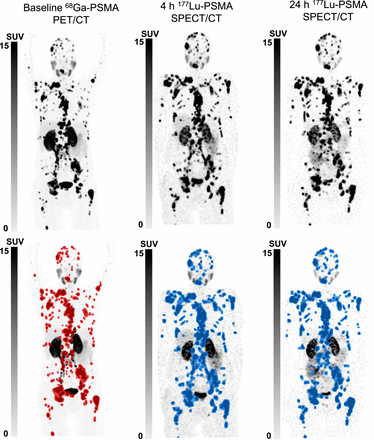
Sites of disease (prostate, bone, lymph node, liver, and lung) were unchanged between the 4- and 24-h [177Lu]Lu-PSMA SPECT/CT and the screening [68Ga]Ga-PSMA-11 PET/CT. Visually, the background activity was higher on the 4-h [177Lu]Lu-PSMA SPECT/CT than on the 24-h [177Lu]Lu-PSMA SPECT/CT or the screening [68Ga]Ga-PSMA-11 PET/CT (Figs. 1 and 2). Eleven of 21 patients (52%) had lesions that were not seen on the 4-h [177Lu]Lu-PSMA SPECT/CT images but were seen on 24-h [177Lu]Lu-PSMA SPECT/CT images. All lesions were of small volume (<2 cm) (Fig. 3A). The median number of missed lesions on 4-h [177Lu]Lu-PSMA SPECT/CT compared with 24-h [177Lu]Lu-PSMA SPECT/CT was 1 (IQR, 0–1). This comprised a total of 20 lesions: 15 (75%) osseous and 5 (25%) nodal. No visceral lesions were missed. The median SUVmax of the missed lesions was 5 (IQR, 4–8). No new lesions identified on the 4-h [177Lu]Lu-PSMA SPECT/CT were not also identified on the 24-h [177Lu]Lu-PSMA SPECT/CT.
From left to right, maximum-intensity projections of screening [68Ga]Ga-PSMA-11 PET/CT, 4-h [177Lu]Lu-PSMA SPECT/CT, and 24-h [177Lu]Lu-PSMA SPECT/CT (top row) and corresponding quantitative maximum-intensity projections (bottom row) of 63-y-old man with mCRPC to regional and distant lymph nodes and widespread osseous metastases showing identical disease distribution on all studies both visually and quantitatively.
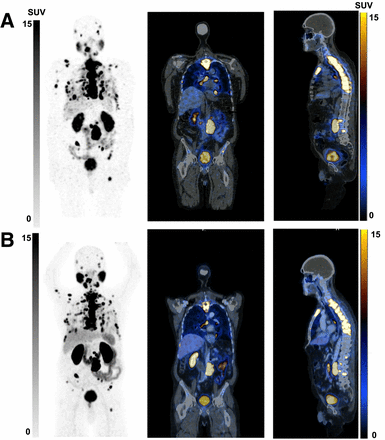
From left to right, maximum-intensity projections, coronal images, and sagittal images of screening [68Ga]Ga-PSMA-11 PET/CT (B) and 4-h [177Lu]Lu-PSMA SPECT/CT (A) of 83-y-old man with mCRPC to retroperitoneal and mediastinal lymph nodes and multiple osseous and bilateral pulmonary lesions. Good image quality is seen at 4-h [177Lu]Lu-PSMA SPECT/CT, with disease distribution identical to that on screening [68Ga]Ga-PSMA-11 PET/CT.
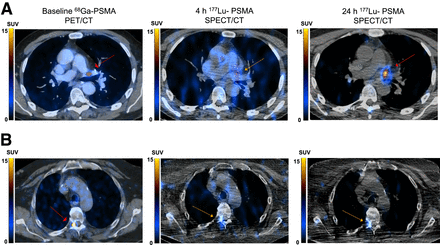
From left to right, transaxial screening [68Ga]Ga-PSMA-11 PET/CT, 4-h [177Lu]Lu-PSMA SPECT/CT, and 24-h [177Lu]Lu-PSMA SPECT/CT. (A) Small left hilar lymph node was missed on 4-h [177Lu]Lu-PSMA SPECT/CT (orange arrow) compared with 24-h [177Lu]Lu-PSMA SPECT/CT and screening [68Ga]Ga-PSMA-11 PET/CT (red arrows). (B) Small osseous lesion was missed on in right pedicle of T4 on 4- and 24-h posttherapy [177Lu]Lu-PSMA SPECT/CT (orange arrows) compared with [68Ga]Ga-PSMA-11 PET/CT (red arrow) (B).
Fourteen patients (67%) had lesions on [68Ga]Ga-PSMA-11 PET/CT but not on either the 4-h and or the 24-h [177Lu]Lu-PSMA SPECT/CT. The median number of missed lesions on posttherapy [177Lu]Lu-PSMA SPECT/CT at 4 h compared with the reference [68Ga]Ga-PSMA-11 PET/CT was 3 (IQR, 0–5). In all these patients, the missed lesions were small (<2 cm) (Fig. 3B).
Comparison of Quantitative Biomarkers of 4- and 24-Hour [177Lu]Lu-PSMA SPECT/CT
Lesion count plus semiautomated quantitative TTV, SUVmax, and whole-body SUVmean for screening [68Ga]Ga-PSMA-11 PET/CT and both 4- and 24-h [177Lu]Lu-PSMA SPECT/CT are summarized in Table 2, with 95% limits of agreement based on difference calculations listed in Supplemental Table 1 (supplemental materials are available at http://jnm.snmjournals.org).
Quantitative Biomarkers on Screening [68Ga]Ga-PSMA-11 PET/CT and 4- and 24-Hour [177Lu]Lu-PSMA-I&T SPECT/CT
The TTV concordance correlation between the 4- and 24-h [177Lu]Lu-PSMA SPECT/CT was substantial (concordance correlation coefficient, 0.988; 95% CI, 0.979–0.997). Although the mean difference 95% CI did not include zero (21–141, 4-h TTV higher), on inspection of the difference Bland–Altman plot (Fig. 4A), it appeared that the spread of values increased as the mean TTV increased. The 95% upper limit of agreement (ratio) regression line was observed to exceed acceptable bounds (Fig. 4E), whereas the lower limit was acceptable at higher TTVs.
(A–D) Bland–Altman plots of difference in 4-h and 24-h [177Lu]Lu-PSMA SPECT/CT TTV (A), number of lesions (B), SUVmax (C), and SUVmean (D), with dashed horizontal 95% limits of agreement and solid line and cap indicating mean difference with 95% CI. (E–H) Bland–Altman plots of ratio in 4-h and 24-h [177Lu]Lu-PSMA SPECT/CT TTV (E), number of lesions (F), SUVmax (G), and SUVmean (H), with dashed regression-derived limits of agreement lines and solid regression-derived bias line. Both axes are on log scale. Shaded area indicates acceptable agreement ratio. ρc = concordance correlation coefficient.
Number of lesions, SUVmax, and whole-body SUVmean concordance correlations were almost perfect or substantial (concordance correlation coefficients of 1.0, 0.988, and 0.988, respectively), and the mean differences were not clinically significant (Figs. 4B–4D). The regression-derived limits of agreement were fully within satisfactory bounds for lesion count and SUVmean for the entire range of relevant values but were in these bounds only for values of SUVmax above the median (Figs. 4F–4H).
ΔTTV Between Dose 1 and 2 [177Lu]Lu-PSMA SPECT/CT and Patient Outcomes
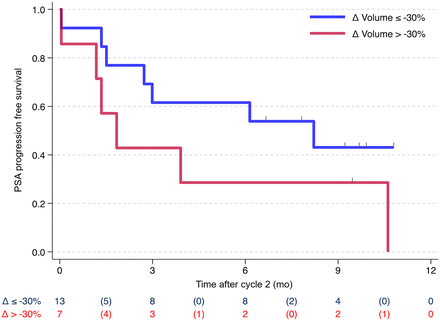
Of the 7 patients with a ΔTTV of more than −30%, 2 achieved PSA-50 (29%), versus 10 of 13 (77%) with a ΔTTV of −30% or less. There were 13 patients with PSA progression or death recorded by July 2024. The median time from cycle 2 was 3.9 mo overall, 1.8 mo in those with a ΔTTV of more than −30%, and 8.2 mo in those with a ΔTTV of −30% or less (Fig. 5).
Kaplan–Meier plots (log-rank test) for PSA progression-free survival by ΔTTV (Δ Volume).
DISCUSSION
Prostate theranostics is improving outcomes in men with mCRPC, and [177Lu]Lu-PSMA-617 is now a standard-of-care therapy after androgen receptor pathway inhibitor and first-line taxane chemotherapy. [177Lu]Lu-PSMA-617 has been administered as 6 doses every 6 wk in phase III randomized trials, without interim imaging with PSMA PET/CT or [177Lu]Lu-PSMA SPECT/CT as imaging response biomarkers (1,2). However, there is increasing evidence that imaging response biomarkers help stratify patients into responders and nonresponders and will play an important role in improving personalization of treatment decisions in prostate theranostics (11,13). Optimizing the cost-effectiveness and convenience of imaging response biomarkers will aid their introduction into routine clinical care. To our knowledge, this was the first study to evaluate the potential of [177Lu]Lu-PSMA SPECT/CT at 4 h for treatment response. The study found that although small lesions were missed on the 4-h compared with the 24-h [177Lu]Lu-PSMA SPECT/CT, likely because of the higher background activity, there was substantial concordance in disease distribution and quantitative parameters. Moreover, the ΔTTV on 4-h [177Lu]Lu-PSMA SPECT/CT at 6 wk predicted patient outcomes.
Imaging of interim response biomarkers has previously been demonstrated to improve treatment response in lymphoma, with an interim 3-mo [18F]F-FDG PET/CT scan stratifying patients into responders and nonresponders and allowing personalized treatment intensification (14). In prostate cancer, the use of both interim (3-mo) PSMA PET/CT and [177Lu]Lu-PSMA SPECT/CT has also been shown to effectively stratify patients into responders and nonresponders (3,11). This ability to identify responders and nonresponders improves further when PSA response is combined with molecular imaging response. Composite biomarker-guided treatment using the 6-wk [177Lu]Lu-PSMA SPECT/CT and PSA response predicts both overall and progression-free survival in prostate cancer (10,11). Patients with an increase in their TTV on the 6-wk [177Lu]Lu-PSMA SPECT/CT and increased PSA or new non–PSMA avid disease on diagnostic CT had an overall survival of 11 mo, compared with 20 mo in those patients with a marked reduction in their TTV and PSA at 6 wk after commencing [177Lu]Lu-PSMA therapy (11). Similarly, the current study showed that patients with a 30% or greater reduction in the TTV on 4-h [177Lu]Lu-PSMA SPECT/CT had a higher PSA-50 and longer PSA progression-free survival of 8 mo compared with 2 mo.
To date, most publications and trial protocols evaluating [177Lu]Lu-PSMA SPECT/CT as a response biomarker have used SPECT imaging acquired 24 h after injection of [177Lu]Lu-PSMA therapy (6,15). Neubauer et al. showed that combined semiquantitative TTV on 48-h [177Lu]Lu-PSMA SPECT/CT and PSA are prognostic for PSA progression-free and overall survival (10). The 24-h time point has been preferred for treatment response assessment as it gives time to clear background activity but without increased scatter due to reduced counts. However, imaging at 24 h is inconvenient and expensive both in time and money for patients, necessitating a return for imaging the day after treatment. The new-generation multidetector cadmium-zinc-telluride–based SPECT/CT has shown prognostic value for changes in TTV on SPECT/CT undertaken as early as 1 h after [177Lu]Lu-PSMA administration (16). This study on standard SPECT/CT cameras demonstrated that visual interpretation is not substantially different between the 4- and 24-h [177Lu]Lu-PSMA SPECT/CT, with some smaller lesions not identified. This difference in number of lesions identified indicates that a serial imaging comparison would need to be performed on scans from consistent time points (either 4 or 24 h).
Pathmanandavel et al. compared semiquantitative TTV on the first-dose 24-h [177Lu]Lu-PSMA SPECT/CT and the screening [68Ga]Ga-PSMA-11 PET/CT in a prospective 56-patient trial and demonstrated an excellent correlation (9). The current study showed a similar disease distribution on posttherapy [177Lu]Lu-PSMA SPECT/CT at both 4 and 24 h compared with the screening [68Ga]Ga-PSMA-11 PET/CT despite some missed small lesions on [177Lu]Lu-PSMA SPECT/CT given its lower spatial resolution. Moreover, the current study showed substantial correlation between the 4- and 24-h [177Lu]Lu-PSMA SPECT/CT in number of lesions, TTV, SUVmax, and SUVmean. However, agreement between the 4- and 24-h image TTV was beyond acceptable limits at lower TTVs. Comparison of TTV between the 4- and 24-h [177Lu]Lu-PSMA SPECT/CT time points may lead to a misestimation of volume change. This finding reinforces the need for serial [177Lu]Lu-PSMA SPECT/CT at consistent time points, either 4 h or 24 h, if it is to be used for treatment response.
The current study was limited by its small sample size. Additionally, this was a single-center study, and acquisition parameters for posttherapy [177Lu]Lu-PSMA SPECT/CT might differ between institutions. The use of [177Lu]Lu-PSMA-I&T as the only [177Lu]Lu-PSMA radiopharmaceutical in the study is another potential limitation, although, the results should remain applicable to [177Lu]Lu-PSMA-617 and other[177Lu]Lu radiopharmaceuticals. A first dose of 8.5 GBq was used in all patients in this analysis as per the TheraP trial; a lower dose than this might have affected the number of lesions identified. However, the value of serial SPECT/CT in the estimation of treatment response is unlikely to have been affected (1).
CONCLUSION
[177Lu]Lu-PSMA SPECT/CT at 4 h after therapy injection appears a promising alternative to 24-h [177Lu]Lu-PSMA SPECT/CT for treatment response assessment despite some variations in the quantitative TTV and missing of a few small lesions. Moreover, it has potential for improved patient convenience and lower cost.
DISCLOSURE
No potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: Can posttherapy [177Lu]Lu-PSMA SPECT/CT acquired 4 h after injection be used for assessment of treatment response?
PERTINENT FINDINGS: Four-hour [177Lu]Lu-PSMA SPECT/CT showed a disease distribution similar to that of 24-h [177Lu]Lu-PSMA SPECT/CT and screening [68Ga]Ga-PSMA-11 PET/CT. Moreover, there was substantial concordance between the quantitative parameters on 4- and 24-h [177Lu]Lu-PSMA SPECT/CT, although several small lesions were not identified. The ΔTTV on 4-h [177Lu]Lu-PSMA SPECT/CT predicted patient outcomes.
IMPLICATIONS FOR PATIENT CARE: [177Lu]Lu-PSMA SPECT/CT at 4 h after injection appears a promising alternative to 24-h [177Lu]Lu-PSMA SPECT/CT for treatment response assessment, with improved patient convenience.
ACKNOWLEDGMENTS
We thank the patients and the clinical staff at the Department of Theranostics and Nuclear Medicine at St Vincent’s Hospital, Sydney, for their support.
Footnotes
Published online Oct. 30, 2024.
- © 2024 by the Society of Nuclear Medicine and Molecular Imaging.
REFERENCES
- Received for publication February 12, 2024.
- Accepted for publication September 30, 2024.