Visual Abstract
Abstract
Neuroendocrine tumor (NET) metastases to the heart are found in 1%–4% of NET patients and have been reported primarily in the form of individual cases. We investigated the prevalence, clinical characteristics, imaging features, and outcomes of NET patients with cardiac metastases on 68Ga-DOTATATE PET/CT. Methods: 68Ga-DOTATATE PET/CT of 490 consecutive patients from a single institution were retrospectively reviewed for sites of metastases. The cumulative cardiovascular event rate and overall survival of patients with cardiac NET metastases (CNMs) were compared with those of a control group of metastatic NET patients without cardiac metastases. In patients with CNMs, the cardiac SUVmax with and without normalization to the myocardial background uptake was compared with a separate cohort of 11 patients with active cardiac sarcoidosis who underwent 68Ga-DOTATATE PET/CT for research purposes. Results: In total, 270 patients with metastatic NETs were identified, 9 (3.3%) of whom had CNMs. All 9 patients had grade 1–2 gastroenteropancreatic NETs, most commonly from the small intestine (7 patients). The control group consisted of 140 patients with metastatic grade 1–2 gastroenteropancreatic NETs. On Kaplan–Meier analysis, there was no significant difference in the risk of cardiovascular adverse events (P = 0.91 on log-rank test) or mortality (P = 0.83) between the metastatic NET patients with and without cardiac metastases. The degree of cardiac DOTATATE uptake was significantly higher in CNMs than in patients with cardiac sarcoidosis without overlap, in terms of both cardiac SUVmax (P = 0.027) and SUVmax–to–myocardial background ratio (P = 0.021). Conclusion: Routine 68Ga-DOTATATE PET/CT can be used to identify CNMs in 3% of patients with metastatic NETs. CNMs do not confer added cardiovascular or mortality risk. A distinguishing feature of CNMs is their high degree of DOTATATE uptake compared with focal myocardial inflammation.
Neuroendocrine tumors (NETs) represent a heterogeneous group of neoplasms that originate from neuroendocrine cells throughout the body (1). Gastroenteropancreatic (GEP) NETs and pulmonary carcinoid tumors account for 87%–95% of NET cases, and the remaining cases arise from unknown or various rare primary sites (1–3). Approximately 20%–30% of NET patients have distant metastases at the time of diagnosis, primarily in the liver and lymph nodes (1,4,5). The bones, lungs, and peritoneum represent less common (<20%) metastatic sites, and rare (<5%) metastatic sites include the brain, ovaries, and heart (4,6–9).
Cardiac NET metastases (CNMs) are estimated at 1%–4% in frequency and are nearly always found in patients with noncardiac metastases (10). They are generally incidentally discovered, but affected patients may present with dyspnea, chest pain, and arrhythmias (11,12). Although asymptomatic patients can be followed without specific therapy, surgical excision may be required for symptomatic CNMs (9,11). Use of external-beam radiotherapy has also been described, and medical therapy such as antiarrhythmic drugs may be considered as well (9,13,14). However, the natural prognosis of patients with CNMs remains unknown. There is only a small body of literature in which patients with CNMs have been followed for outcome evaluation, attributed to the rarity of CNMs and difficulty in their identification (11).
CNMs have been classically identified using echocardiography, with MRI as a superior modality for detailed evaluation (9,11). In the absence of dedicated cardiac imaging, CNMs are difficult to identify on conventional CT (15). The current use of somatostatin receptor (SSTR) PET as the standard of care for functional imaging of NET patients has improved detection of CNMs (15,16). Unlike 18F-FDG cardiac PET imaging, which requires fasting or a ketogenic diet for evaluation of pathologic myocardial uptake, abnormal cardiac uptake on SSTR PET can be identified even on routine staging studies (17,18). However, abnormal cardiac uptake on SSTR PET is not uniquely attributable to metastases, as it has been described in cases of cardiac inflammation such as sarcoidosis, myocarditis, and the postinfarction state (19–21). A method to distinguish between metastatic and inflammatory cardiac uptake on SSTR PET has not, to our knowledge, been reported to date.
In the present study, we used 68Ga-DOTATATE PET/CT, a type of SSTR PET, to identify cardiac metastases in NET patients. We examined the prevalence of CNMs and the prognosis of patients with CNMs. Finally, we compared the degree of cardiac DOTATATE uptake in patients who had CNMs with a well-characterized external cohort of patients who had active cardiac sarcoidosis to distinguish between metastatic and inflammatory DOTATATE uptake in the heart.
MATERIALS AND METHODS
Study Population
Approval from the University of Pennsylvania Institutional Review Board was obtained before the study, and the requirement for informed consent was waived. The medical records of 490 consecutive patients who underwent 68Ga-DOTATATE PET/CT for their clinical care at the University of Pennsylvania Health System from December 2016 to September 2018 were retrospectively reviewed. In total, 270 patients with metastatic NETs were included in the study.
Image Acquisition and Analysis
The patients underwent 68Ga-DOTATATE PET/CT according to the standard institutional protocol. At least 4 wk after the last somatostatin analog (SSA) therapy, patients were given intravenous administration of 68Ga-DOTATATE at a dose of 2 MBq/kg up to 200 MBq. Static PET images were acquired from the skull base to mid thigh 50–70 min after the radiotracer administration. Low-dose unenhanced CT was performed for attenuation correction and anatomic colocalization. MIM version 7 (MIM Software Inc.) was used for image processing and analysis.
To identify the patients with cardiac metastases, each patient’s 68Ga-DOTATATE PET/CT was individually reviewed for sites of metastases. Cardiac SUVmax was recorded by placing a spheric region of interest at the area of myocardium with the highest 68Ga-DOTATATE uptake, with and without normalization to the mean myocardial background SUV in the left ventricle (LV). SUV was corrected for total body weight.
Patient Assessments
The baseline clinical, laboratory, and pathologic characteristics of the patients were collected, as well as echocardiography results when available. Clinical follow-up data were reviewed for cardiovascular adverse events and overall survival, measured with reference to the date of 68Ga-DOTATATE PET/CT. Cardiovascular adverse events were defined as new episodes of heart failure hospitalization, ventricular tachycardia, atrial fibrillation/flutter, stroke, or transient ischemic attack. All-cause mortality was also captured.
Statistical Analysis
Based on the identified cohort of NET patients with cardiac metastases (cardiac metastasis group), a control group of metastatic NET patients without cardiac metastases was defined (noncardiac metastasis group). Laboratory values were compared between the 2 groups using the unpaired t test. Cardiovascular adverse events and overall survival between the cardiac metastasis group and noncardiac metastasis group were compared using Kaplan–Meier survival analysis with the Mantel–Cox test. Using the unpaired t test, cardiac SUVmax was compared between the cardiac metastasis group and a separate cohort of 11 patients with active cardiac inflammation secondary to cardiac sarcoidosis (inflammation group). Patients in the inflammation group prospectively underwent 68Ga-DOTATATE PET/CT at our institution for research purposes, with cardiac 18F-FDG PET/CT for verification of active cardiac inflammation (19).
All statistical tests were 2-sided, and P values of less than 0.05 were considered statistically significant. The statistical analyses were performed using Prism 8 (GraphPad Software).
RESULTS
Patient Characteristics
Nine patients with 19 CNMs were identified, representing 3.3% of those with metastatic NETs. The baseline characteristics of the patients are summarized in Table 1. Seven (78%) patients were male, and the median age was 61 y (range, 54–77 y). All 9 patients had grade 1–2 GEP NETs, most commonly from the small intestine (78%). The CNMs were most commonly solitary (56%) but could involve any location of the heart (Table 2). The CNMs were not identified or suspected on routine transthoracic echocardiography, available for 6 patients. Noncardiac metastases were also present in all 9 patients, mostly involving the liver (78%) and lymph nodes (78%). Hypertension (67%) and diabetes mellitus (44%) were the most common comorbidities. The patients were most commonly treated with SSAs (67%).
Baseline Characteristics of Study Population
Number and Location of Cardiac Metastases
For outcome comparison, we identified a control group of 140 grade 1–2 metastatic GEP NET patients with available clinical follow-up data (Fig. 1). Compared with the noncardiac metastasis group, the cardiac metastasis group showed a higher proportion of men (78% vs. 46%) and cardiovascular comorbidities (22). The treatment history and echocardiogram results were comparable between the 2 groups. Reduced baseline cardiac function, when present, was not attributed to prior NET treatments.
Flow diagram for patient inclusion in study. G1/2 = grade 1 or 2.
The 2 groups also showed an overall similar biochemical evaluation. The cardiac metastasis group included 4 (44%) patients with a history of carcinoid syndrome (CS) but none with carcinoid heart disease (CHD). The noncardiac metastasis group included 75 (54%) patients with a history of CS and 2 (1%) patients with CHD. There was no significant difference in the levels of serum serotonin (597 ± 303 vs. 443 ± 531 ng/mL, P = 0.52), serum chromogranin A (786 ± 1,230 vs. 1,720 ± 6,156 ng/mL, P = 0.71), or 24-h urine 5-hydroxyindoleacetic acid (28 ± 29 vs. 16 ± 23 mg/d, P = 0.31) between the 2 groups (mean ± SD, cardiac metastases vs. control groups).
Outcomes in NET Patients with Cardiac Metastases
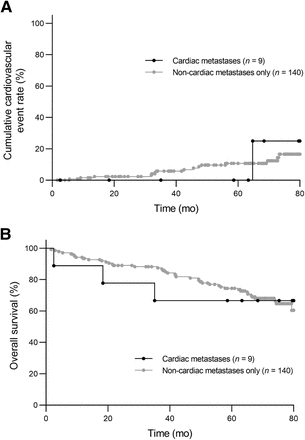
The median duration of clinical follow-up for the 149 patients was 67.5 mo (range, 1.6–82.6 mo). Only a single cardiovascular adverse event (atrial fibrillation) occurred in the cardiac metastasis group. In the noncardiac metastasis group, the number of cardiovascular adverse events was 3 for heart failure admission, 1 for nonsustained ventricular tachycardia, 4 for atrial fibrillation or flutter, and 8 for stroke or transient ischemic attack in a total of 15 (11%) patients. There was no significant difference in the risk of cardiovascular adverse events between the 2 groups (P = 0.91, Fig. 2A). The overall mortality rate was 33% in the cardiac metastasis group and 29% in the noncardiac metastasis group, without significant differences in overall survival (P = 0.83, Fig. 2B). Four patients with 6 CNMs had follow-up 68Ga-DOTATATE PET/CT. During the follow-up period (range, 41–80 mo), 2 cases of extracardiac disease progression and peptide receptor radionuclide therapy (PRRT) were identified (Fig. 3). Otherwise, all 4 patients showed stable disease on SSA monotherapy. The cardiac metastases remained stable in number and DOTATATE uptake, even in the setting of extracardiac disease progression or PRRT. Pre- versus post-PRRT cardiac function evaluation was not performed, but no PRRT-related cardiotoxicity was observed clinically.
No significant difference in cardiovascular event rate (A) or overall survival (B) was observed between metastatic grade 1 or 2 GEP NET patients with and without cardiac metastases (P > 0.05).
Follow-up of 6 cardiac metastases in 4 patients with multiple 68Ga-DOTATATE PET/CT, whose disease remained stable on SSA monotherapy unless otherwise indicated. Cardiac metastases were stable in number and DOTATATE uptake, even in setting of extracardiac disease progression or PRRT.
Metastatic Versus Inflammatory Cardiac DOTATATE Uptake
Examples of metastatic and inflammatory DOTATATE uptake in the heart are shown in Figure 4, where a NET metastasis at the LV apex shows increased DOTATATE uptake compared with active cardiac sarcoidosis at the interventricular septum. The degree of focal cardiac DOTATATE uptake in the cardiac metastasis group was significantly higher, without overlap, than in the inflammation group, in terms of both cardiac SUVmax (P = 0.027) and SUVmax–to–LV background ratio (P = 0.021) (Fig. 5). In our study population, threshold values of 4 for cardiac SUVmax and 3.5 for SUVmax–to–LV background ratio could distinguish between cardiac metastases and inflammation with 100% accuracy.
NET metastasis at left ventricular apex (arrows) shows higher 68Ga-DOTATATE uptake than in active cardiac sarcoidosis at interventricular septum (circles) in another patient. SUVmax–to–LV background ratio is 6.5 vs. 3.0.
Cardiac SUVmax (A) and SUVmax–to–LV background ratio (B) in NET compared with active cardiac sarcoidosis. Degree of focal cardiac uptake secondary to metastatic NET was significantly higher without overlap with sarcoidosis (P < 0.05).
DISCUSSION
In this study, we examined 68Ga-DOTATATE PET/CT in 490 patients to identify 9 patients with CNMs. Our prevalence of 3.3% for CNMs, of 270 patients with metastatic NETs, falls within the previously reported range of 1%–4% (10,18,23,24). Although there are larger cross-sectional studies on the prevalence of CNMs on SSTR PET (18,23,24), our study contributes to the existing literature by reporting the patients’ clinical outcomes and by differentiating cardiac DOTATATE uptake between CNM and myocardial inflammation.
Interestingly, all patients with CNMs in our study had GEP NETs, mostly from the small intestine. Although lung cancer is the most common source of cardiac metastases, accounting for 36%–39% of cases (25), pulmonary carcinoid tumors rarely metastasize to the heart. For example, pulmonary carcinoid tumors accounted for only 4 (9%) of 45 of CNM cases based on a review by Jann et al. (11), although they represent 20%–30% of NET cases (1). More recently, Calissendorff et al. reported a 4.3% rate of CNMs on 68Ga-DOTATOC PET/CT when only ileal NET patients were evaluated, which is higher than the reported rate of 0.83% by Carreras et al. for a general SSTR PET cohort (15,18). Although the biologic basis for this behavior remains unknown, identification of cardiac metastases may suggest a small-bowel primary in a case of widely metastatic NET with unknown primary site.
Our finding that CNMs do not lead to added cardiovascular or mortality risk suggests their indolent nature, supporting active surveillance as a management option in asymptomatic patients (26,27). Previously, several retrospective studies suggested that CNMs have limited clinical impact (16,26,27). In a review of 45 CNM cases, only 2 (4%) patients had life-threatening complications due to involvement of the conducting system by CNMs (11). In our literature review of 27 nonsurgically managed CNM cases with clinical follow-up (Table 3), we also found 2 cases of fatal ventricular arrhythmias. The overall mortality in the literature cohort was 15% over the median follow-up of 20 mo, in relative agreement with our CNM cohort mortality (33% over 46 mo). Given that patients with NETs often have a long predicted survival and the risk of fatality from CNMs is nonzero, some asymptomatic patients may still undergo surgical excision of their CNMs (15). Notably, the presence of CNMs does not preclude patients from receiving the standard-of-care systemic therapy for metastatic NETs such as SSA and PRRT (15,28).
Published Cases on Nonsurgically Managed CNMs with Follow-up Mortality Outcomes
With increasing use of PRRT for metastatic NETs, its use in patients with CNMs has been also reported. We found in the literature 45 CNM patients who received PRRT, as summarized in Table 4. Stable disease or a partial response is the expected outcome in CNMs after PRRT. 177Lu-DOTATATE uptake in CNMs can be measured over time with quantitative SPECT/CT (29), which allows for tumor dosimetry. The reported assessment of PRRT-associated cardiotoxicity in patients with CNMs is heterogeneous, ranging from clinical observation to cardiac MRI before and after therapy (30). The available short-term data suggest that PRRT is generally well tolerated in patients with CNMs without cardiotoxicity, including in a retreatment setting (31). However, one patient showed persistent suppression of ejection fraction (from 65% to 34% over the follow-up period of 3 y) associated with PRRT (32), and 2 other patients showed mild pericarditis treated with oral steroids (27). Given such cases, some authors recommend cardiac function evaluation before and after PRRT (32) or even prophylactic steroid pretreatment in patients with CNMs (33). Although data on long-term cardiotoxicity after PRRT of CNMs are yet to be available, long-term cardiotoxicity is unlikely given the radioresistance of the heart relative to PRRT tumor dosimetry (34,35).
Published Journal Articles on PRRT in Patients with CNMs
None of our patients with CNMs had CHD, despite some with a history of CS, consistent with recent PET studies on CNMs with a pooled CHD prevalence of 4% (2/51 patients) (23,26,36,37). In contrast, older literature reported a higher CHD prevalence of 36%–73% in the setting of CNMs (11,38), which is likely attributed to an echocardiographic diagnosis of CNMs only in patients with a high metastatic burden, as well as lack of SSA therapy. SSA therapy has shown approximately 70% effectiveness in treating CS, with an associated prevention of CHD (39). Although metastatic NET in general poses a risk of developing CS and CHD, prior studies suggest that CNMs do not specifically increase the risk of CS or CHD compared with noncardiac metastases (23,36,37). For example, in a recent study of 276 patients with CS, 104 of whom had CHD, the prevalence of CNMs was 3%, similar to the general NET cohort (40). The comparable biochemical profile we found between the cardiac metastases and the control groups supports the lack of a major influence of CNMs on the hormonal pathophysiology of metastatic NET. Therefore, patients with CNMs would not require additional evaluation for CS and CHD beyond what is recommended for metastatic NET patients.
We found a significantly higher level of DOTATATE uptake in CNM than in active inflammation, with an uptake threshold that yielded 100% accuracy in distinguishing between the two. Our results are in keeping with previous studies that reported only low-level cardiac uptake on SSTR PET secondary to inflammation (19–21). Pathologically, inflammatory macrophages display only weak SSTR expression in contrast to the strong SSTR expression seen in NETs (41,42). Outside our study population, there may still be an overlap between metastatic and inflammatory cardiac uptake on SSTR PET for 2 reasons. First, the partial-volume effect may artificially lower the observed uptake of small CNMs (43). Second, NETs may exhibit low to absent uptake on SSTR PET, often seen in high-grade or aggressive tumors (44–46). In clinical practice, interpretation of focal cardiac uptake on SSTR PET would be further improved by taking into account the clinical history and other available diagnostic and radiologic information. However, our findings indicate that in patients with NET metastases, focally increased DOTATATE uptake in the heart (particularly ≥3 times background activity) is most likely the result of metastatic disease rather than inflammation.
Once CNMs are identified on SSTR PET, cardiac MRI may be used for further anatomic characterization including lesion size, location, and extent of myocardial involvement (36). Since cardiac MRI is less sensitive and its independent interpretation identifies only 56% of the lesions found on SSTR PET, cardiac MRI should be compared with SSTR PET and the 2 studies may be retrospectively fused for direct comparison (36). Cardiac MRI, along with echocardiography, also provides an evaluation of cardiac function. Routine echocardiography is highly insensitive (0%–18%) for identification of CNMs, consistent with our results, and its role is limited to evaluation of cardiac function and CHD (26,27,36). Standard contrast-enhanced CT is also insensitive (19%) for evaluation of CNMs (24). In addition to the 68Ga-based SSTR PET we used, other SSTR-targeted radiotracers such as 64Cu-DOTATATE and 18F-SiTATE can also reveal CNMs (29,47). It remains to be seen whether the shorter positron range of the newer SSTR PET agents offers improved detection of CNMs (48). Beyond SSTR PET, CNMs have also been identified using 18F-fluorodopa, 18F-FDG, and 123I-iobenguane, but their sensitivity for NETs is lower than that of SSTR PET (37,44,49–52). In particular, cardiac metastases may be challenging to identify on 18F-FDG PET because of background myocardial uptake (53). At present, SSTR PET remains the best modality for initial identification of CNMs, and subsequent evaluation and management may be guided by multimodality imaging as well as clinical evaluation.
The main limitation of the present study is the absence of histologic proof that the lesions identified by 68Ga-DOTATATE PET/CT represent CNMs. Histologic proof is generally not available in studies on CNMs because of the invasiveness of cardiac biopsy and high radiologic diagnostic confidence (24,27,37). Another limitation is the small number of patients with CNMs for outcome analysis. The outcome results are supported by our literature review, but the literature cases could not be directly combined with our study cohort because of their heterogeneity in pathology, follow-up, and reported outcomes. The size of the CNMs could not be measured because of their poor visualization on routine oncologic CT and MRI, and dedicated cardiac CT or MRI was not available in most of our CNM cases. Baseline troponin and natriuretic peptide levels were not available in most patients since the measured values were generally in acute care settings. Since approximately 10% of NET patients have DOTATATE-negative disease (54), the actual prevalence of CNMs may be underestimated in our study. In such patients, our uptake threshold for metastatic versus inflammatory cardiac DOTATATE uptake would not be valid as previously mentioned. As differences in image acquisition and processing methods can produce variability in SUV measurements (55), the proposed threshold for metastatic versus inflammatory uptake may need to be adjusted. Given the inherent limitations of a single-center retrospective study (56,57), our results would benefit from validation in a larger study with respect to both clinical outcomes and distinction from inflammatory cardiac uptake.
CONCLUSION
Routine 68Ga-DOTATATE PET/CT can be used to identify CNMs in 3% of patients with metastatic NETs, most commonly from the small intestine. CNMs do not confer added cardiovascular or mortality risk, supporting active surveillance as a management option in asymptomatic cases. A distinguishing feature of CNMs is their high degree of DOTATATE uptake compared with focal myocardial inflammation. The optimal surveillance protocol and management strategy for CNMs remain to be investigated.
DISCLOSURE
The study was partly funded by the University of Pennsylvania Research Foundation and the Radiologic Society of North America (RSD1903). Hwan Lee is funded by the National Institutes of Health 5-T32-EB004311-19 grant. Daniel Pryma discloses research funding from Lantheus, Point Biopharma, and Fusion Pharmaceuticals; honoraria from Molecular Targeting Technologies Inc.; and stock options from Molecular Targeting Technologies Inc. and Trevarx. No other potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: How can CNMs be identified on 68Ga-DOTATATE PET/CT, and what are their implications?
PERTINENT FINDINGS: On 68Ga-DOTATATE PET/CT, cardiac metastases can be identified as areas of focal myocardial uptake in 3% of metastatic NET patients. Metastatic NET patients with and without cardiac metastases show a comparable cardiovascular event rate and overall survival. Cardiac metastases demonstrate significantly higher DOTATATE uptake compared with inflammatory activity from active cardiac sarcoidosis.
IMPLICATIONS FOR PATIENT CARE: Cardiac metastases can be reliably identified on 68Ga-DOTATATE PET/CT and do not carry added cardiovascular or mortality risk.
Footnotes
Published online Oct. 3, 2024.
- © 2024 by the Society of Nuclear Medicine and Molecular Imaging.
REFERENCES
- Received for publication April 14, 2024.
- Accepted for publication September 9, 2024.