Visual Abstract
Abstract
Cancer of unknown primary (CUP) represents a heterogeneous group of metastatic tumors for which standardized diagnostic work-up fails to identify the primary site. We aimed to describe the Peter MacCallum Cancer Centre experience with 18F-FDG PET/CT in extracervical CUP with respect to detection of a primary site and its impact on management. A secondary aim was to compare overall survival (OS) in patients with and without a detected primary site. Methods: CUP patients treated between 2014 and 2020 were identified from medical oncology clinics and 18F-FDG PET/CT records. Information collated from electronic medical records included the suspected primary site and treatment details before and after 18F-FDG PET/CT. Clinicopathologic details and genomic analysis were used to determine the clinically suspected primary site and compared against 2 independent masked reads of 18F-FDG PET/CT images by nuclear medicine specialists to determine sensitivity, specificity, accuracy, and the rate of detection of the primary site. Results: We identified 147 patients, 65% of whom had undergone molecular profiling. The median age at diagnosis was 61 y (range, 20–84 y), and the median follow-up time was 74 mo (range, 26–83 mo). Eighty-two percent were classified as having an unfavorable CUP subtype as per international guidelines.18F-FDG PET/CT demonstrated a primary site detection rate of 41%, resulted in a change in management in 22%, and identified previously occult disease sites in 37%. Median OS was 16.8 mo for all patients and 104.7 and 12.1 mo for favorable and unfavorable CUP subtypes, respectively (P < 0.0001). Median OS in CUP patients when using 18F-FDG PET/CT, clinicopathologic, and genomic information was 19.8 and 8.5 mo when a primary site was detected and not detected, respectively (P = 0.016). Multivariable analysis of survival adjusted for age and sex remained significant for identification of a potential primary site (P < 0.001), a favorable CUP (P < 0.001), and an Eastern Cooperative Oncology Group status of 1 or less (P < 0.001). Conclusion: 18F-FDG PET/CT plays a complementary role in CUP diagnostic work-up and was able to determine the likely primary site in 41% of cases. OS is improved with primary site identification, demonstrating the value of access to diagnostic 18F-FDG PET/CT for CUP patients.
Cancer of unknown primary (CUP) is a heterogeneous group of metastatic tumors for which standardized diagnostic work-up fails to identify the site of origin at diagnosis (1,2). Although accounting for only 3%–5% of all malignancies, it represents the sixth most common cause of cancer death in Australia, with an estimated incidence of 8.2 per 100,000 (1,2). Median overall survival (OS) for CUP remains poor, at 9–12 mo (3), with a 5-y OS rate of only 14% (2). The European Society of Medical Oncology (ESMO) CUP guidelines (1) categorize CUP into 2 subtypes: favorable and unfavorable (Table 1). The favorable subtype accounts for 15%–20% of patients, with tumors amenable to long-term control if treated similarly to tumors of known origin with a similar presentation (e.g., CUP with a colorectal immunohistochemistry profile managed like colorectal cancer) in a multidisciplinary setting. The remaining 80%–85% of tumors are considered an unfavorable subtype, with a dismal prognosis of less than 12 mo. A palliative platinum-based chemotherapy regimen is the standard treatment and is reserved for patients with a good performance status; however, complete or partial response rates are seen in only 20%–40% of patients (1,4,5).
CUP Subtype According to ESMO Guidelines
Current guidelines for CUP recommend that patients initially undergo a thorough physical examination, basic blood tests, pathologic review, and CT of the chest, abdomen, and pelvis (1,6). Appropriate sex-specific investigations (i.e., breast ultrasound/mammogram, testicular ultrasound, prostate-specific antigen) and tailored investigations (i.e., gastroscopy/endoscopy) may also be required depending on the clinical picture. The National Comprehensive Cancer Network guidelines (6) make recommendations focused on the histologic subtype and clinical presentation, whereas the ESMO (1) and Optimal Care Pathway guidelines (3) intend to rapidly identify treatable patient subsets and an occult primary lesion through a rational, focused approach. Despite improvement in conventional diagnostic processes, the primary site is identified before death in less than 30% of CUP patients (2). Although not mandated in all current guidelines outside of head and neck CUP, PET/CT is increasingly used in many centers, with the most used PET radiotracer being the glucose analog 18F-FDG. It provides a noninvasive nuclear medicine imaging technique to help identify primary malignant tumors and the extent of metastatic disease. Systematic reviews and metaanalyses performed to date recommend 18F-FDG PET/CT in the diagnostic work-up of CUP patients despite the fact that most CUP studies have had heterogeneous patient populations and no standardized diagnostic process (7–11). Prior review of 18F-FDG PET/CT in 31 CUP patients at Peter MacCallum Cancer Centre suggested a possible primary in 61% of cases, although this was confirmed in only 26%. Management was changed after 18F-FDG PET/CT in 38% of cases (12).
We provide an update on the clinical utility of 18F-FDG PET/CT for CUP patients who were treated through a specialized CUP clinic, using modern genomic analysis of many cases. Retrospective analysis was performed to assess the diagnostic yield by determining the number of cases in which a likely primary site was determined by 18F-FDG PET/CT, as well as the impact on treatment decisions. Second, we sought to examine OS in patients for whom a potential primary site was determined, compared with patients for whom a primary site was not determined.
MATERIALS AND METHODS
This retrospective analysis was performed to evaluate the Peter MacCallum Cancer Centre experience with 18F-FDG PET/CT in CUP patients with respect to detection of a primary site and its impact on management between 2014 and 2020. Patients were identified from medical oncology clinics and the PET/CT database. The study was approved by the Human Research Ethics Committee of Peter MacCallum Cancer Centre, and the requirement to obtain informed consent was waived.
Inclusion criteria were as follows: biopsy-proven malignancy before 18F-FDG PET/CT with disseminated disease; no history of malignancy in the prior 3 y other than nonmelanoma skin lesions, cervical carcinoma in situ, or breast carcinoma in situ; and a minimum of 12 mo of follow-up data in the medical records. Patients with isolated metastatic tumor deposits to the head and neck region were excluded.
The demographic data that were collected included age at diagnosis and sex. The clinicopathologic information that was collected included histologic subtype and differentiation, CUP subtype as per ESMO guidelines (1), date of biopsy, performance status, smoking status, genomic analysis (if known), the most likely primary site based on histopathologic and clinical assessment before and after 18F-FDG PET/CT as documented in medical records, number of organs involved, major metastatic site of involvement, treatment decision before and after 18F-FDG PET/CT, number of investigations performed, date of last follow-up, and date and cause of death.
PET scans were obtained on one of three 3-dimensional scanners: Siemens Biograph 64, GE HealthCare Discovery 690, and GE HeathCare Discovery 710 for 90 patients scanned at Peter MacCallum Cancer Centre. The 18F-FDG dose was 3.6 MBq/kg. Patients were prepared by fasting for a minimum of 4 h before isotope injection and encouraged to drink only water in the interim. The uptake phase was 60–75 min, during which the patient rested supine. A whole-body (base of brain to pelvis) attenuation-corrected study was generally acquired with the arms elevated. Fifty-seven patients underwent 18F-FDG PET/CT in an outside institution, and these were imported to a PACS for review.
18F-FDG PET/CT reads were performed independently by 2 masked nuclear medicine specialists; discordant results were reviewed, with a consensus primary site determined. Clinic notes, histopathologic results, and treatment response, along with genomic data (if available; included gene expression profiling and next-generation sequencing), were reviewed to determine the likely primary site. 18F-FDG PET/CT was classified as follows: true-positive (when 18F-FDG PET/CT detected the primary tumor and was confirmed by histopathology [through repeat biopsy/resection] or by clinical follow-up); probably positive (when 18F-FDG PET/CT was suggestive of the primary and was confirmed by the clinical course without definitive diagnosis); false-positive (when 18F-FDG PET/CT detected the primary tumor and was not confirmed by histopathology or by clinical follow-up); true-negative (when 18F-FDG PET/CT did not detect the primary tumor and it remained unknown in clinical follow-up); or false-negative (when 18F-FDG PET/CT did not detect the primary tumor but it was confirmed by histopathology or by clinical follow-up). One of the difficulties with CUP is that the primary site is not always confirmed by histopathology; therefore, the true-positive cases and probably positive cases were combined when determining sensitivity, specificity, accuracy. and detection rate.
Baseline demographics were summarized using descriptive statistics. Categoric variables were summarized and reported using counts and percentages. Sensitivity, specificity, accuracy, and detection rate (detection rate = [true-positive + probably positive]/all patients) were calculated for 18F-FDG PET/CT. The Cohen κ-concordance score was calculated to determine interreader variability. OS was defined as the date from diagnosis to the date of death or last known follow-up.
All patients were included in analyses. OS was described using Kaplan–Meier methods, and we compared survival between favorable and unfavorable CUP subtypes; dominant metastatic sites in the unfavorable CUP subtype; extent of disease (solitary, locoregional, and distant); site-specific and empiric chemotherapy; potential primary and no primary identified on the basis of 18F-FDG PET/CT alone and on the basis of a combination of clinical, genomic, and 18F-FDG PET/CT results; and whole-body metabolic tumor volume (wbMTV) above and below the median wbMTV. Cox proportional hazards models were used to assess the impact of potential prognostic factors on OS. All analyses were performed using Prism version 9.5.1 (GraphPad) and R Studio version 1.4.1717.
RESULTS
Baseline Demographics and Clinical Characteristics of Patients
In total, 310 patients were reviewed in the CUP clinic at Peter MacCallum Cancer Centre between July 2014 and August 2020; for these patients, 206 18F-FDG PET/CT scans were available for review; 147 patients met the inclusion criteria and were included (Fig. 1). Age ranged from 20 to 84 y, with a median of 61 y. Most tumors were adenocarcinoma (54%), with lymph nodes being the dominant site of disease in 51 patients (35%). Ninety-three percent of patients were of good performance status (Eastern Cooperative Oncology Group status, 0–1), and 120 patients (82%) were classified as having unfavorable CUP as per the ESMO guidelines (1). Forty-four patients (30%) had a first-degree relative with a history of cancer, and 26 patients (18%) had a prior cancer history (Table 2).
Consolidated Standards of Reporting Trials (CONSORT) diagram. ECOG = Eastern Cooperative Oncology Group status.
Patient Characteristics
There was no standardized diagnostic work-up performed among the different histologic subtypes. Fifty-three patients (36%) had only CT of the chest, abdomen, and pelvis before the 18F-FDG PET/CT, 4 patients (3%) had 18F-FDG PET/CT as their first investigation, and 90 patients (61%) had a combination of imaging modalities (including ultrasound, MRI, whole-body bone scanning, and mammography). Thirty-six patients (24%) had 3 or more investigations before 18F-FDG PET/CT. The detection rate was 20% in patients who had only CT of the chest, abdomen, and pelvis, compared with 18% for those who underwent multiple investigations before 18F-FDG PET/CT.
Independent Masked 18F-FDG PET/CT Reads
Two nuclear medicine specialists performed independent masked reviews of the 147 18F-FDG PET/CT scans: one a nuclear medicine–trained radiologist with 4 y of experience in 18F-FDG PET/CT reading, and the other a nuclear medicine physician with 20 y of experience in 18F-FDG PET/CT and 10 y of experience in stand-alone 18F-FDG PET. The detection rate was 26% and 39% for readers 1 and 2, respectively. There were 53 cases (36%) that had a discordant primary-site determination between the 2 reviewers, and a further read was performed by both nuclear medicine specialists to reach consensus about the potential primary site in all cases. The level of agreement between the 2 reviewers was fair as calculated by a Cohen κ-coefficient of 0.26.
Consensus 18F-FDG PET/CT Read Results
18F-FDG PET/CT suggested a primary site in 61 patients (41%); however, only 14 (10%) were histologically proven and defined as true-positive. Forty-seven (31%) 18F-FDG PET/CT scans were deemed probably positive on the basis of the clinical information or molecular analysis. Thirty-one patients (21%) had a potential primary site identified by 18F-FDG PET/CT; however, these were not proven histologically or by molecular analysis or during clinical follow-up and were thus classified as false-positive. Fifteen (48%) of the 31 patients remained classified as having unresolved CUP. In 16 patients (11%), the primary site was not suggested on 18F-FDG PET/CT or confirmed histologically or by molecular analysis and remained unknown in clinical follow-up; these cases were classified as true-negative. The 18F-FDG PET/CT did not detect a primary site in 39 patients (27%); however, these cases were confirmed histologically or by clinical follow-up or molecular analysis and were classified as false-negative. Of the 39 patients, the 2 most common potential primary sites were the lower gastrointestinal in 11 patients (28%) and the lung in 9 patients (23%). In this study, 18F-FDG PET/CT had a sensitivity of 61%, specificity of 34%, accuracy of 52%, and detection rate of 41% (Table 3). When compared with the original 18F-FDG PET/CT reports, which were performed with access to clinical information, 7 (5%), 22 (15%), 16 (11%), 75 (51%), and 27 (18%) cases were classified as true-positive, probably positive, false-positive, false-negative, and true-negative, respectively, with sensitivity of 28%, specificity of 63%, accuracy of 38%, and a detection rate of 20%.
Diagnostic Utility of 18F-FDG PET/CT
Disease Characteristics and Treatment
In 54 patients (37%), the 18F-FDG PET/CT upstaged their disease with the detection of occult disease. Thirty-four patients (23%) had a change in management as a result of the 18F-FDG PET/CT, most commonly resulting in a change to palliative-intent treatment (15/54, 28%) (Table 4). Median SUVmax was 9.9 (range, 2.8–42.6), and median wbMTV was 142 cm3 (range, 0.2–4,506.7 cm2) for all CUP patients.
Change in Treatment Because of 18F-FDG PET/CT Results
One hundred seventeen patients (80%) had distant disease, with 21 (14%) and 9 (6%) having locoregional and solitary disease, respectively. Seventy-eight patients (53%) had molecular profiling performed. The results of molecular profiling influenced tissue-of-origin determination and subsequent management in 27 patients (28%), with 15 cases (56%) being due to gene expression profiling, 9 (33%) to next-generation sequencing, and 3 (11%) to both gene expression profiling and next-generation sequencing.
Seventy-nine patients (54%) received site-specific therapy, 41 patients (28%) received empiric chemotherapy, and 27 patients (18%) received no systemic therapy. Of the patients who did not receive systemic therapy, 14 (52%) received best supportive care and 6 (22%) received palliative radiotherapy. Five patients (19%) underwent curative-intent surgery, and 2 patients (7%) received curative-intent radiotherapy. Of the patients who received site-specific therapy, 17 (22%), 17 (22%), and 16 (20%) received treatment targeting the lung, lower gastrointestinal tract, and upper gastrointestinal tract, respectively.
OS
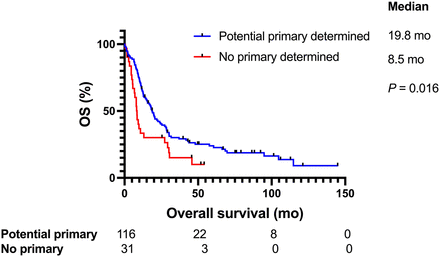
The median follow-up time was 76 mo, and the median OS of all CUP patients was 16.8 mo (95% CI, 12.6–22). The median OS for favorable-subtype CUP patients was 104.7 mo, compared with 12.1 mo for unfavorable-subtype CUP patients (P < 0.0001) (Fig. 2). When looking at the unfavorable CUP subtype based on the dominant metastatic site determined by SUV, we found that patients who presented with lymph node disease had a median OS of 30.5 mo, compared with 11.3, 11, 9.9, 9.3, and 20.6 mo for patients who presented with lung, bone, peritoneum, liver, and other, respectively (P < 0.0001). In CUP patients with solitary disease, the median survival was not reached but was 57.6 and 12.6 mo with patients with locoregional and distant disease, respectively (P < 0.0001). In CUP patients who received site-specific therapy, the median OS was 22 mo, compared with 15.2 mo in those who received empiric chemotherapy (P = 0.05) (Fig. 3). In CUP patients for whom a potential primary site was detected on 18F-FDG PET/CT (true-positive and probably positive cases), the median OS was 18.8 mo, compared with 18.3 mo in patients without a primary site detected (true-negative cases) (P = 0.81). The false-positive and false-negative cases were excluded because the primary site was either not detected or incorrectly detected by 18F-FDG PET/CT. The median OS in CUP patients when 18F-FDG PET/CT, clinicopathologic information, and genomic information was used was 19.8 versus 8.5 mo in patients with a primary site detected and not detected, respectively (P = 0.016) (Fig. 4). CUP patients with a wbMTV above the median had a shorter median OS than did those with one below the median, at 28.4 and 10.5 mo, respectively (P < 0.0001) (Fig. 5). A multivariable analysis of survival adjusted for age and sex remained significant for the identification of a potential primary site (P < 0.001), a favorable CUP (P < 0.001), and an Eastern Cooperative Oncology Group status of 1 or less (P < 0.001).
Favorable vs. unfavorable CUP subtype (Wilcoxon test).
Site-specific vs. empiric chemotherapy (Wilcoxon test).
Identification of potential primary site using 18F-FDG PET/CT, clinical information, and genomics guiding treatment (Wilcoxon test).
CUP patients with wbMTV above and below median wbMTV (Wilcoxon test).
DISCUSSION
In our retrospective analysis of 147 CUP patients who underwent 18F-FDG PET/CT as part of their diagnostic work-up, we observed a 41% primary site detection rate by a consensus read, although with only fair interreader concordance. An improved OS was observed in CUP patients for whom a potential primary site was detected and was concordant with clinical and genomic results. Our results provide further evidence that unfavorable-CUP patients have a worse prognosis; however, patients presenting with lymph node–dominant disease have a better median OS. Occult disease was detected in 37% of cases, and there was a change in management in 23% of patients because of the 18F-FDG PET/CT, providing incremental value beyond simply detection of a primary site.
Multiple studies evaluating the utility of 18F-FDG PET/CT in cervical lymph node metastasis in CUP patients have demonstrated its utility in detecting a primary tumor as well as occult metastases (7,13,14). In extracervical CUP (Table 5), a literature review of 152 patients from 4 retrospective studies had a 39.5% detection rate of primary tumor site, and pooled estimates for sensitivity, specificity, and accuracy were 87%, 88%, and 87.5%, respectively (15). Significant heterogeneity in inclusion criteria and diagnostic work-up was observed among the 4 studies. Soni et al. performed a retrospective analysis on 83 CUP patients with a detection rate of 39% and with sensitivity, specificity, and accuracy of 89%, 85%, and 87%, respectively (16). In our study, the detection rate was 41%, similar to prior studies; however, the sensitivity, specificity, and accuracy were 60%, 34%, and 52%, respectively, which are lower than previously published. This reflects a cohort of patients who have been heavily investigated with conventional diagnostic methods and modern genomic sequencing without a primary site evident, making the detection of a primary site by 18F-FDG PET/CT less likely, as reported by Soni et al. (16).
Extracervical CUP Studies
Woo et al. performed a systematic review and metaanalysis on the impact of 18F-FDG PET/CT on the management of 2,795 CUP patients; in this study, the pooled proportion of patients with management changes was 35% (9). Interestingly the reason for the change in management was mainly detection of the primary site (22%), and only 14% had detection of occult disease. Reinert et al. reported on 155 CUP patients; a primary tumor was detected in 23.3% of patients on 18F-FDG PET/CT, and 26.5% of patients had a major change in their intended treatment after 18F-FDG PET/CT (17). Burglin et al. revealed a pooled detection rate of 40.93% in their systematic review and metaanalysis of 18F-FDG PET/CT in CUP patients with extracervical metastases (10). Subramaniam et al. reported a 43.1% change in management in the CUP cohort, and further testing was avoided in three fourths of patients (18). In our study, 18F-FDG PET/CT detected a potential primary site in 60 patients (41%), with a change in management in 34 (23%) and detection of occult disease in 54 (37%). These results are consistent with published results and demonstrate the benefit of 18F-FDG PET/CT over conventional imaging methods in determining the extent of disease.
To date, interobserver variability has been examined with respect to 18F-FDG PET/CT parameters, staging, response to treatment, and cancer screening (19,20). In our study, 46% of cases had a discordant primary site initially determined by the nuclear medicine specialists; however, a consensus was reached for all patients. In the presence of widespread metastases, assigning a lesion as a primary as opposed to another metastatic site can be difficult and often will be based on pattern recognition, which is favored by experience, or on morphologic features on correlative CT, which may be favored by formal radiology training. However, adding clinicopathologic data can also help differentiate between 2 differential diagnoses—for example, for ovarian lesions as being primary tumors or as being part of a peritoneal metastatic process. These considerations highlight the importance of multidisciplinary review and discussion of CUP cases because of the heterogeneous nature of the disease pattern.
Improved histopathologic diagnosis and the use of genomic data can help determine the likely primary site of CUP tumors and may have therefore resulted in the higher false-positive and false-negative rate in our study than in other 18F-FDG PET/CT studies. Genomic methods are evolving, with RNA tests having been the most widely used to date; however, 2 recent randomized control trials have demonstrated no survival benefit from site-specific chemotherapy compared with empiric chemotherapy (21,22). Multiomic approaches combining DNA and RNA features are also improving specificity, with interpretation of cancer genome features helping to resolve cancer type (23). The efficacy of molecularly guided therapy in CUP patients was recently demonstrated in the international multicenter CUPISCO trial (11). In our study, patients who received site-specific therapy based on all available diagnostic information had a longer median OS than did patients who received empiric chemotherapy (22 vs. 15.2 mo, P = 0.02). Use and access to next-generation sequencing in a timely manner to complement 18F-FDG PET/CT is paramount to assist with diagnostic and treatment decisions in CUP patients.
Limitations to our analysis are its retrospective nature, the rarity of CUP, and the limited patient numbers; however, this was a relatively large patient cohort compared with those in the existing literature. Including CUP patients who have had an extensive diagnostic work-up introduces selection bias. Strengths of this analysis are its use of clinical, genomic, and imaging data to determine the most likely primary site in order to determine patient outcomes.
CONCLUSION
18F-FDG PET/CT plays a complementary role in the diagnostic work-up of CUP patients along with histopathologic, radiologic, and genomic data. There was a 41% primary site detection rate with 18F-FDG PET/CT. Improved OS with tissue of origin identification demonstrates the value of access to diagnostic 18F-FDG PET/CT for CUP patients.
DISCLOSURE
No potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: What is the utility of 18F-FDG PET/CT in the diagnostic work-up of CUP patients?
PERTINENT FINDINGS: There is a 41% primary site detection rate with 18F-FDG PET/CT, and the median OS is longer in patients when a primary site is identified using 18F-FDG PET/CT, clinical, and genomic information.
IMPLICATIONS FOR PATIENT CARE: 18F-FDG PET/CT plays a complementary role in the diagnostic work-up of CUP patients.
Footnotes
Published online Sep. 5, 2024.
- © 2024 by the Society of Nuclear Medicine and Molecular Imaging.
REFERENCES
- Received for publication February 22, 2024.
- Accepted for publication August 13, 2024.