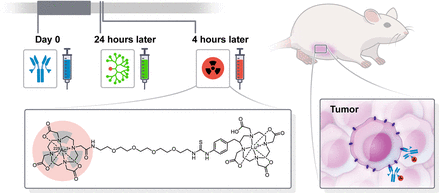
Visual Abstract
Abstract
Radioimmunotherapy using 225Ac, a highly cytotoxic α-particle emitter, has potential for treating advanced breast cancer, especially human epidermal growth factor receptor 2 (HER2)–positive cases. We use a pretargeted radioimmunotherapy (PRIT) approach consisting of a 3-step intravenous regimen (step 1: bispecific anti-HER2/anti-DOTA antibody; step 2: clearing agent; step 3: 225Ac-radiolabeled Proteus DOTA, or [225Ac]Ac-Pr). Our goal was to establish curative 225Ac-PRIT with high therapeutic indices. Methods: The impact of [225Ac]Ac-Pr specific activity was evaluated in the BT-474 breast xenograft model. We tested the effects of [225Ac]Ac-Pr dosing during PRIT on tumor-targeting efficiency and tissue biodistribution. Using a 225Ac-PRIT regimen consisting of a ratio of 1.19 nmol of bispecific antibody to 0.60–0.66 nmol of [225Ac]Ac-Pr, we evaluated therapy in the BT-474 model and a patient-derived xenograft model. BT-474-tumor–bearing mice were treated with 1 or 2 cycles of 225Ac-PRIT (37 kBq/cycle) separated by 1 wk. A dose escalation study was performed on the BT-474 model to establish an absorbed radiation dose of approximately 40 Gy (relative biological effectiveness [RBE], 5) as a nephrotoxic dose, as no such histologic findings were observed in prior studies at the 20.7-Gy (RBE, 5) renal dose level. Results: In the BT-474 model, 100% (20/20) achieved complete responses and histologic cure in 17 of 20 (85%) of the treated animals. One-cycle and 2-cycle treatments were equally effective. Treatments were well tolerated, with no chronic radiation toxicity documented during necropsy at 175 d. Dosimetry estimates (RBE, 5) per 37 kBq administered for tumors and kidneys were 210 and 3.5 Gy, respectively. In the patient-derived xenograft model, a single 225Ac-PRIT treatment led to 60% (3/5) complete response and prolonged survival (>93 d) versus no treatment (30 d; P = 0.0185). Lastly, a 225Ac-PRIT regimen was identified that induces severe chronic nephrotoxicity (41.4 Gy/592 kBq; RBE, 5). Conclusion: Safe and effective 225Ac-PRIT regimens were developed in 2 preclinical models of advanced HER2-positive human breast cancer with tumor cure without dose-limiting nephrotoxicity. This study establishes crucial preclinical dosimetry benchmarks for 225Ac-PRIT and provides a compelling rationale for its advancement into the clinic.
Advanced metastatic breast cancer has a poor prognosis, especially for highly aggressive triple-negative and human epidermal growth factor receptor 2 (HER2)–positive subtypes (1). HER2 is an oncogene that is overexpressed in 15%–20% of breast cancers and is a clinically established therapeutic target (2,3). Although targeted therapies such as the HER2-targeted monoclonal antibody–drug conjugate trastuzumab–deruxtecan have improved outcomes, challenges remain because of treatment-related adverse events and tumor resistance to HER2 blockade in HER2-positive advanced-stage breast cancer (2).
Radioimmunotherapy, which combines therapeutic radionuclides with monoclonal antibodies to cancer-associated cell surface antigens, has therapeutic potential for many cancer types (4). HER2 is a promising target for radioimmunotherapy with the α-particle–emitting radionuclide 225Ac, as HER2-mediated internalization of the radioimmunoconjugate can potentially lead to increased retention of highly cytotoxic α-particles generated during 225Ac decay (half-life, 9.92 d) (5). It has been shown that the anti-HER2 humanized monoclonal IgG antibody trastuzumab radiolabeled with 225Ac is a potent therapeutic agent against HER2-positive breast cancer (6).
To enhance the therapeutic indices (TIs; tumor–to–normal-tissue absorbed dose ratios) of radioimmunotherapy using IgGs, a promising alternative strategy is to use a multistep or pretargeting approach (4). Various strategies have been explored for pretargeted radioimmunotherapy (PRIT), including with bispecific antibody (BsAb) (7). We established an anti-HER2 PRIT method consisting of administration of an anti-HER2/anti-DOTA hapten BsAb (an IgG single-chain variable fragment consisting of trastuzumab and the anti-DOTA[metal] complex single-chain variable fragment C825) followed by a clearing agent and 177Lu-labeled aminobenzylDOTA for delivery of therapeutic β-particle radiation (8). For 225Ac-therapy, we use Proteus DOTA (Pr), consisting of an empty DO3A-chelate for 225Ac, tethered via a short polyethylene glycol linker to a lutetium-complexed DOTA for picomolar C825 binding (as [225Ac]Ac-Pr) (9). We have also confirmed that Pr can be radiolabeled with its imaging surrogate, 111In (as [111In]In-Pr) (9). Notably, all radiolabeled forms of Pr contain stable lutetium (175Lu)-complexed aminobenzylDOTA as an affinity handle for direct binding to C825 (Fig. 1).
Schematic representation of 3-step HER2-targeted 225Ac-PRIT including chemical structure of [225Ac]Ac-Pr. For [111In]In-Pr, 225Ac is replaced with 111In.
This study aimed to establish high-TI 225Ac-PRIT for HER2-expressing breast cancer. Building on the work of Cheal et al. (9), we have significantly enhanced the specific activity of [225Ac]Ac-Pr (∼6-fold) (10). During the preliminary studies of HER2-pretargeted [225Ac]Ac-Pr, we observed moderate efficacy in the HER2-positive BT-474 breast cancer model (9). In the current study, our hypothesis was that we would see improved efficacy, including TI, based on the specific activity of the [225Ac]Ac-Pr. We took this opportunity to investigate how the administered mass of [225Ac]Ac-Pr influences tumor uptake and normal-tissue biodistribution in the BT-474 model during 225Ac-PRIT. We also investigated the kinetics of BsAb-pretargeted internalization of [111In]In-Pr as a surrogate for [225Ac]Ac-Pr, as internalization can significantly impact the potency of 225Ac targeted α-therapy (5). Dosimetry calculations were performed to establish dose–response and dose–toxicity relationships to correlate with antitumor efficacy and toxicities. The 225Ac-PRIT regimen was validated in a HER2-positive breast cancer patient–derived xenograft model.
MATERIALS AND METHODS
General details regarding cell lines, PRIT reagents, and radiolabeling of Pr can be found in the supplemental materials (available at http://jnm.snmjournals.org).
Mouse Models and PRIT Regimen
All animal procedures were performed in compliance with Memorial Sloan Kettering Cancer Center’s institutional Animal Care and Use Committee guidelines. The BT-474 model was established according to previously described procedures in female athymic nude mice (nu/nu) (9). Additional details are provided in the supplemental materials. A HER2-positive breast cancer patient–derived xenograft (M37 (12)) was established from fresh surgical specimens with Memorial Sloan Kettering Cancer Center Institutional Review Board approval in female BALB/c Rag2−/−IL-2Rγc−/− mice.
The PRIT regimen was as follows for all biodistribution and therapy studies: the BsAb (0.25 mg, 1.19 nmol carrying 2.4 nmol of DOTA-[radio]hapten binding capacity) was administered at −28 h, followed by a clearing agent (25 µg, 2.76 nmol) at −4 h and [225Ac]Ac-Pr (as indicated below) at 0 h (Fig. 1) (11). Selection of this regimen was based on prior studies with 177Lu-PRIT in the BT-474 model (8).
Cellular Binding and Internalization Assay
To assay the internalization kinetics of BsAb-pretargeted [111In]In-Pr, a method based on the work of Heskamp et al. (13) was used. We did not determine the internalization kinetics of BsAb-pretargeted [225Ac]Ac-Pr. Instead, following Kondo et al. (6), we assumed that substituting 225Ac for 111In would not alter these properties. Additionally, we presumed that the subcellular distribution of [111In]In-Pr and [225Ac]Ac-Pr in BT-474 cells would be similar, as suggested by Kondo et al. (14). More details are provided in the supplemental materials.
Biodistribution and Dosimetry
In the BT-474 model, we compared the biodistribution profiles of 225Ac-PRIT with 2 different administered [225Ac]Ac-Pr molar doses. Groups of tumor-bearing mice were treated with BsAb and clearing agent and were injected with [225Ac]Ac-Pr at either 296 kBq (26.9 nmol; n = 6) or 37 kBq (0.60 nmol; n = 5). The higher dose was previously used to establish both efficacy and toxicity, whereas the lower dose was expected to be subsaturating in terms of absolute tumor uptake of Pr (9). After 24 h, the mice were euthanized for an ex vivo biodistribution assay. In an additional experiment, we performed serial biodistribution with 225Ac-PRIT in groups of BT-474 tumor–bearing mice (3–5/group; 37 kBq, 0.60 nmol) at 2, 24, 72, 192, and 240 h after injection to calculate dosimetry. A relative biological effectiveness (RBE) of 5 was used for α-radiation as suggested by Sgouros et al. (15). More details are provided in the supplemental materials.
Radiopharmaceutical Therapy
Two separate studies were performed with BT-474 tumor–bearing mice (denoted as experiments 1 and 2). A single study was performed with M37 tumor–bearing mice (denoted as experiment 3). Supplemental Table 1 shows the starting tumor volumes and treatment group sizes (5–10/group) for all studies. During experiment 1, groups of BT-474 tumor–bearing mice were treated with 1 or 2 cycles of 225Ac-PRIT (cycle 1: 37 kBq, 0.64 nmol; cycle 2: 37 kBq, 0.66 nmol) separated by 1 wk. This regimen was based on prior studies showing no acute toxicity after 225Ac-PRIT (9). During each cycle, BsAb, clearing agent, and [225Ac]Ac-Pr were administered. During experiment 2, groups of BT-474 tumor–bearing mice were treated with 1 cycle of anti-HER2 BsAb only. Survival was defined as time to tumor doubling and included deaths from other causes. These study endpoints were based on prior studies with 177Lu-PRIT in the BT-474 model, which can have relatively slow growth kinetics (8). For experiment 1 only, surviving animals were submitted alive at 175 d to the Laboratory of Comparative Pathology of Memorial Sloan Kettering Cancer Center for pathologic analysis, hematology, and serum chemistry (the supplemental materials provide the anatomic and clinical pathology methods).
During experiment 3, groups of M37 tumor–bearing mice were treated with 1 cycle of 225Ac-PRIT (37 kBq, 0.64 nmol). A multicycle 225Ac-PRIT regimen was not attempted in this model. Treatment controls included [225Ac]Ac-Pr only or 225Ac-PRIT with control BsAb (antiglycoprotein A33 [GPA33]/anti-DOTA (16)) in place of anti-HER2 BsAb. Also, a biodistribution study (5 mice/group) was performed at 24 h after injection to confirm tumor targeting during 225Ac-PRIT. Survival was defined as the time to a tumor diameter of 10 mm and included deaths from other causes. No postmortem pathologic analysis, hematology, or serum chemistry was performed.
A complete response is defined as a tumor volume of 4.2 mm3 or smaller. A cure is defined as the absence of tumor cells at the implantation site, as confirmed by histological analysis.
Dose Escalation Study to Evaluate Potential Nephrotoxicity
A dose escalation study was performed on a group of BT-474 tumor–bearing mice (n = 10) with treatment consisting of 2 cycles of 225Ac-PRIT separated by 1 wk (cycle 1: 296 kBq, 19.4 nmol; cycle 2: 296 kBq, 27.1 nmol). This treatment regimen delivered an estimated kidney-absorbed dose of 41.4 Gy. A nontreatment control group (n = 8) was included for comparison. Groups were monitored for 167 d after treatment initiation, and 6 randomly selected survivors from each group were submitted for necropsy and pathologic evaluation of toxicity. Antitumor effects were not monitored during this study.
Data Analysis
Quantitative data were expressed as mean ± SD unless otherwise noted. Statistical and area-under-the curve analyses were performed using GraphPad Prism 9.3.1. Kaplan–Meier survival curves were analyzed with the Mantel–Cox test. Two-sided Student t tests were calculated, and a P value of less than 0.05 was considered to be statistically significant.
RESULTS
In Vitro Binding and Internalization of BsAb-Pretargeted [111In]In-Pr
Cell binding and internalization of BsAb-pretargeted [111In]In-Pr were studied in vitro using BT-474 cell cultures (Fig. 2). After 1 h of 37°C incubation, rapid and efficient cell binding was observed (19.02% ± 3.36% added activity per 106 cells) and [111In]In-Pr was mainly membrane-bound (8%–9% of total cell bound activity internalized). As shown in Figure 2A, peak cell binding was observed after 1 h and remained relatively constant up to 24 h (16.41% ± 7.09% added activity per 106 cells). The internalized fraction gradually increased until 28% of the total cell-associated activity was internalized after 24 h (Fig. 2B). In the absence of BsAb, minimal binding and internalization were observed of [111In]In-Pr (P < 0.0001 for all time points), suggesting that [111In]In-Pr was internalized as the BsAb/[111In]In-Pr complex (Fig. 2B). The percentage internalized activity of total cell-associated activity was 19.3% based on area-under-the-curve analysis.
Internalization of anti-HER2 BsAb/[111In]In-Pr complex by HER2-positive BT-474 human breast cancer cells at 37°C. (A) Total bound and internalized activity of anti-HER2 BsAb + [111In]In-Pr. (B) Percentage internalized activity of total cell-bound for anti-HER2 BsAb + [111In]In-Pr or [111In]In-Pr control. ****P < 0.0001.
Optimization of Administered [225Ac]Ac-Pr During 225Ac-PRIT
As a first step, we conducted a comparative biodistribution study on groups of BT-474 tumor–bearing mice to evaluate the effect of [225Ac]Ac-Pr mass during 225Ac-PRIT. These data are presented in Figures 3A and 3B and Supplemental Table 2. In summary, with the lower administered [225Ac]Ac-Pr mass (0.60 vs. 26.9 nmol), we observed significantly higher tumor uptake (10.48 ± 3.66 percentage injected activity [%IA]/g vs. 0.38 ± 0.20 %IA/g; P = 0.0001) at 24 h after injection. However, there was also significantly higher blood uptake (0.68 ± 0.11 %IA/g vs. 0.03 ± 0.01 %IA/g; P < 0.0001) and comparable kidney uptake (0.63 ± 0.16 %IA/g vs. 0.47 ± 0.04 %IA/g; P = 0.0322). Consequently, the 2 225Ac-PRIT regimens resulted in similar tumor-to-blood ratios (15.4 vs. 12.7), but the low-mass dose regimen showed a significantly improved tumor-to-kidney uptake ratio (16.6 vs. 0.8), suggesting a more favorable TI for the kidney. The underlying mechanism is that during 225Ac-PRIT, the target has a saturable receptor density, and the amount of administered [225Ac]Ac-Pr mass can have a negative effect on tumor uptake due to saturation of the receptor sites at the targeted cancer cells with nonradioactive Pr.
Influence of [225Ac]Ac-Pr mass dose on in vivo performance of HER2-targeted 225Ac-PRIT system. (A) Shown is ex vivo biodistribution assay 24 h after administration of [225Ac]Ac-Pr (37 kBq, 0.60 nmol; n = 5) or [225Ac]Ac-Pr (296 kBq, 26.9 nmol; n = 6) in groups of nude mice bearing subcutaneous BT-474 xenografts. Data are presented as %IA/g (left ordinate, all tissues) and pmol/g (right ordinate, tumors and kidneys only). Tabulated data and pmol/g for other tissues are provided in Supplemental Table 2. (B) Corresponding tumor–to–normal-tissue ratios. (C) Serial biodistribution data of optimized 225Ac-PRIT. Shown is ex vivo biodistribution assay after administration of [225Ac]Ac-Pr (37 kBq, 0.60 nmol) in groups of nude mice bearing subcutaneous BT-474 xenografts (3–5/group: 3 for 2 h, 5 for 24 and 72 h, and 3 for 192 and 240 h). Tabulated data are provided in Supplemental Table 3. *P < 0.05. **P < 0.01. ***P < 0.001. ****P < 0.0001. Lg = large; n.s. = no significant difference; Sm = small.
Serial Biodistribution and Dosimetry
Figure 3C and Supplemental Table 3 show the time-dependent biodistribution of 225Ac-PRIT (37 kBq, 0.60 nmol) in mice with BT-474 tumor xenografts. Time-integrated activities were calculated from these serial biodistribution data, and murine-specific internal dosimetry was estimated on the basis of total local absorption of α-emissions. Dosimetry for the tumors and kidneys for 225Ac-PRIT (296 kBq, 26.9 nmol) was extrapolated from 225Ac-PRIT (37 kBq, 0.60 nmol) by scaling the 24-h uptake data, assuming similar time–activity curve shapes. Similarly to Konijnenberg et al. (17), we postulate that the clearance kinetics of the [225Ac]Ac-Pr were unaffected by receptor saturation at the tumor. As shown in Figure 3A and Supplemental Table 2, saturation was not likely in the kidneys. As shown in Table 1, the estimated absorbed dose for BT-474 tumors was 33-fold higher during 225Ac-PRIT with [225Ac]Ac-Pr (37 kBq, 0.60 nmol, 5.670 Gy/kBq vs. 26.9 nmol, 0.170 Gy/kBq). The kidney-absorbed doses, however, were comparable (respectively: 0.60 nmol, 0.095 Gy/kBq; 26.9 nmol, 0.070 Gy/kBq). Additional dosimetry is provided in Supplemental Table 4.
Dosimetry of 225Ac-PRIT in BT-474 Model
Radiopharmaceutical Therapy
Treatment of BT-474 tumor–bearing mice with 1 or 2 cycles of 225Ac-PRIT (cycle 1: 37 kBq, 0.64 nmol; cycle 2: 37 kBq, 0.66 nmol) led to significant tumor regression, including complete responses (Fig. 4A). Furthermore, treatment with 225Ac-PRIT showed significantly enhanced survival over nontreated controls and anti-HER2 BsAb-only controls (log-rank, P < 0.0001) (Fig. 4B). The median survival was 25 d, 25 d, and not yet reached (>140 d) for nontreated, anti-HER2 BsAb-only, and 225Ac-PRIT, respectively. No significant difference in median survival was observed between nontreated and anti-HER2 BsAb-only controls (P = 0.2275). Both 1-cycle and 2-cycle treatments produced 100% (20/20) complete responses and histologic cure in 17 of 20 animals, or 85% of the treated animals. The therapy results are summarized in Supplemental Table 5.
225Ac-PRIT treatment of BT-474 human breast cancer cell–derived xenograft. Data are shown as mean ± SE of mean. (A) Tumor growth curves after 225Ac-PRIT treatment. (B) Kaplan–Meier survival curves for pooled data (criterion: time for tumor to double in volume or death for any reason). (C) Normalized body weight changes after 225Ac-PRIT treatment. n = 5 for no treatment experiment 1, and n = 10 for all other treatment groups. Exp = experiment.
For the M37 PDX model, a single 225Ac-PRIT cycle (37 kBq, 0.64 nmol) led to 60% (3/5) complete responses and prolonged survival (>93 d) compared with no treatment (30 d; P = 0.0185), [225Ac]Ac-Pr only (23 d; P = 0.0086), or GPA33 (nonspecific) 225Ac-PRIT (36 d; P = 0.0364). One mouse treated with 225Ac-PRIT could not be located starting at 30 d (tumor of 3.6 mm3 at 23 d). The therapy results are summarized in Supplemental Figure 1 and Supplemental Table 7. A biodistribution assay confirmed efficient tumor targeting during 225Ac-PRIT (Supplemental Table 6).
Toxicity
All treatments in BT-474 tumor–bearing mice were well tolerated, with no acute toxicity. No BT-474 treatment groups showed average weight loss greater than 5% of the pretreatment baseline (Fig. 4C). For M37-treated groups, mild (∼10% change from baseline) but transient weight loss was observed (Supplemental Fig. 2). However, in the [225Ac]Ac-Pr–only and the GPA33 (nonspecific) 225Ac-PRIT control groups, 2 of 5 mice in each cohort were found deceased (cause of death unknown). For the [225Ac]Ac-Pr–only group, this occurred at 16 d despite weight losses of 8% and 10% from pretreatment baseline (tumor volumes at 8 d, 77.8 and 33.6 mm3, respectively). For the GPA33 (nonspecific) 225Ac-PRIT group, this occurred at 23 d despite weight losses of 9% and 4% from pretreatment baseline (tumor volumes at 16 d, 95.1 and 43.5 mm3, respectively).
From the BT-474 study, all 225Ac-PRIT–treated animals and a subset of untreated controls (5 untreated controls and 10 each of 1- and 2-cycle 225Ac-PRIT) were submitted for final necropsy at 175 d. A variety of histopathologic changes were noted; these are presented in Supplemental Table 8. We found no evidence of treatment-related radiotoxicity. A summary of the histologic scoring of renal degenerative tubulointerstitial changes is presented in Table 2. In Figure 5, representative histologic images of kidneys stained with hematoxylin and eosin are shown. No postmortem pathologic analysis was performed during the M37 study.
Histologic Scoring of Renal Degenerative Tubulointerstitial Changes
Representative histologic images of kidneys stained with hematoxylin and eosin. (A) Kidney from untreated control mouse showing normal shape and size (scale bar, 1 mm). (B) Boxed region from A showing minimal alterations, such as presence of amphophilic material within renal tubules (arrows) and interstitial inflammatory infiltrates (arrowhead) (scale bar, 50 µm). (C) Kidney from mouse at 175 d treated with 2 cycles of 225Ac-PRIT separated by 1 wk (cycle 1: 37 kBq, 0.64 nmol; cycle 2: 37 kBq, 0.66 nmol) showing normal shape and size (scale bar, 1 mm). Estimated kidney-absorbed dose is 7.0 Gy. (D) Boxed region from C showing minimal alterations, such as presence of amphophilic material within renal tubules (arrows) (scale bar, 50 µm).
From the BT-474 study, hematology (Supplemental Table 9) and blood chemistry (Supplemental Table 10) were unremarkable in all animals of the different groups. Low creatinine kinase and alkaline phosphatase levels in various animals of different groups are likely artifactual given the lack of consistent morphologic changes in the tissues synthesizing or containing such enzymes. No hematology or serum chemistry was performed during the M37 study.
Dose Escalation Study
Treatment with 2 doses of 296 kBq of 225Ac-PRIT was tolerated without weight loss (Supplemental Fig. 3). A single animal was found dead at 63 d (cause of death unknown), but otherwise there were no unscheduled mortalities.
During necropsy at 167 d, a high incidence of treatment-related renal histopathology was observed (Supplemental Table 11). Significant tubular injury with interstitial fibrosis was observed in 6 of 6 treated mice that underwent necropsy. A summary of the histologic scoring of renal degenerative tubulointerstitial changes is presented in Table 2. Representative histologic images of kidneys stained with hematoxylin and eosin taken from a treated mouse are shown in Supplemental Figure 4. The evidence of renal injury was also observed grossly as pale, small kidneys; decreased kidney weight (range, 0.198–0.378 g; control range, 0.330–0.451 g); and moderately increased blood urea nitrogen and creatinine on serum chemistry (Supplemental Table 12). Hematology was unremarkable in all treated animals (Supplemental Table 13).
DISCUSSION
In this report, we document that when high-specific-activity 225Ac-PRIT (37 kBq/mouse) was administered in the BT-474 model, a tumor-absorbed dose of 210 Gy was curative and a kidney-absorbed dose of 3.5 Gy caused subclinical toxicity without observable changes in blood urea nitrogen or creatinine. In preclinical models of human advanced breast cancer, we achieved efficient tumor targeting combined with high TIs by administering sufficient amounts of both BsAb and [225Ac]Ac-Pr, with a ratio of 1.19 nmol of BsAb (2.4 nmol of DOTA-binding sites) to 0.60–0.66 nmol of [225Ac]Ac-Pr. In prior studies, it was necessary to give significantly higher administered [225Ac]Ac-Pr (296 kBq/mouse, 50.3 Gy; Table 2) to achieve less effective tumor control than in the current studies with high-specific-activity 225Ac-PRIT (37 kBq/mouse, 210 Gy; Table 2). These results show that when the specific activity of [225Ac]Ac-Pr is improved, we can achieve improved TI for kidney (from 2.5 to 60.0) while also significantly improving the tumor-absorbed dose 33-fold from 0.170 to 5.670 Gy/kBq (Table 2).
Here, we also explored a multicycle 225Ac-PRIT regimen. One-cycle and 2-cycle treatments were equally effective. During a 2-cycle treatment (total administered [225Ac]Ac-Pr, 74 kBq/mouse), we estimated an absorbed dose to tumor and kidney of 420 and 7.0 Gy, respectively, with mild renal toxicity observed. This was consistent with our prior studies, which showed mild renal toxicity in mice treated with 296 kBq (9); there, we estimated an absorbed dose to kidney of 20.7 Gy. During the dose escalation study with a cumulative therapeutic activity of 592 kBq/mouse, significant tubular injury with interstitial fibrosis was observed in all animals (kidney dose, 41.4 Gy). Therefore, we conclude that the maximum tolerated dose to kidney for 225Ac-PRIT falls between 20.7 and 41.4 Gy. These findings are consistent with prior work (Table 2).
Effective anti-HER2 225Ac-PRIT may face challenges related to internalization and HER2 resistance. HER2 is known to internalize, and internalization of BsAb during PRIT may impair the intratumoral capture of radioligand, leading to ineffective tumor targeting. Additionally, the internalization kinetics may vary depending on whether the radioligand binds to the BsAb or whether it causes crosslinking of the BsAb on the cell membrane. Nevertheless, we achieved high TIs in blood (TI, 42) and kidney (TI, 60.0) while delivering 5.670 Gy/kBq to the tumor. These TIs are significantly higher than those previously reported during HER2 177Lu-PRIT with the same BsAb in the same model (8) and HER2 225Ac-PRIT on HER2-expressing human ovarian peritoneal xenografts (18). However, in the latter (18), these differences may be attributed to the properties of the xenograft model, the route of administration of BsAb and [225Ac]Ac-Pr, and variations in HER2 expression levels between the BT-474 and SKOV3 cell lines (19). For HER2 177Lu-PRIT (8), differences may be due to the type of clearing agent, type of radioligand (aminobenzyl-DOTA was used to complex 177Lu for 177Lu-PRIT), and administered [177Lu]Lu-aminobenzylDOTA mass. Differences in radioligand type also raise the potential for variations in radioligand-mediated BsAb crosslinking at the membrane. It is anticipated that in both [177Lu]Lu-aminobenzylDOTA and [225Ac]Ac-Pr, the anti-DOTA C825 antibody will bind only to the Lu-aminobenzylDOTA moiety with high affinity. Therefore, these factors should be examined in greater detail to understand how to optimize TIs during HER2 PRIT.
Another potential concern is inefficient BsAb binding to tumor with intrinsic or acquired trastuzumab resistance. In such cases, it may be beneficial to use an alternative anti-HER2 antibody that targets an epitope different from trastuzumab (e.g., HT-19 (20)). These 2 possible mechanisms of resistance and approaches to overcoming them will require additional hypothesis-based experimental data to resolve the issue.
Numerous preclinical investigations of HER2-directed α-radioimmunotherapy in breast cancer have been reported, including with alternative antibody formats to IgGs such as single-domain antibodies and diabodies that are cleared primarily through the kidney (21). Renal retention after targeted radiopharmaceutical therapy can result in high radiation doses and potentially lead to end-stage renal disease (22). The normal-tissue dose limit for kidneys in radiopharmaceutical therapy (toxicity endpoint, end-stage renal disease; toxicity rate, 5%) is 23–26 Gy (∼36-Gy biologically effective dose) (23). Long-term follow-up studies of patients treated with 225Ac-radiopharmaceuticals have shown treatment-related kidney failure, but no dose–toxicity relationships were established (24,25). Our findings then are consistent with these current recommendations for kidney dose tolerances for 225Ac-radiopharmaceutical therapy. We have determined that kidney, and particularly renal proximal tubular cells, are the critical tissue for radiation toxicity for 225Ac-PRIT.
We demonstrated that 225Ac-PRIT was highly efficacious in both the BT-474 model and a HER2-overexpressing patient-derived xenograft model. However, the response rates varied significantly between the 2 models. The difference is unlikely to be due to reduced tumor uptake of [225Ac]Ac-Pr in the patient-derived xenograft model (Supplemental Table 6), as both models show comparable uptake at 24 h after injection. Instead, the intrinsic cellular radiosensitivity may differ. We acknowledge that BT-474 has been shown to be relatively radiosensitive to both low– and high–linear-energy-transfer radiation in comparison to other frequently studied HER2-overexpressing human cancer cell lines (26). Another contributing factor could be the potential differences in immune response due to the different mouse strains and the potential direct immunotherapeutic effects of the trastuzumab portion of the BsAb, which delays growth in the BT-474 model (Fig. 4) and the M37 model (12). Other factors influencing the therapeutic efficacy of targeted α-radiopharmaceutical therapy may include the subcutaneous tumor microenvironment and heterogeneity of dose distribution (27).
On the basis of the clinical history of PRIT and the high TIs reported, 225Ac-PRIT shows significant potential for clinical translation (7). However, the current 3-step approach, although useful as a test system, may pose barriers to clinical translation. To overcome these barriers, we are adapting the self-assembling and disassembling platform to target HER2. This platform has demonstrated the ability to achieve high TIs without requiring a clearing agent (28). Our strategy will be guided by ongoing clinical trials of self-assembling and disassembling PRIT (NCT05130255 and NCT05994157) and the recently completed trial of HER2-directed α-radioimmunotherapy (NCT04147819). PRIT has significant advantages with respect to TI that are likely to be needed for meaningful efficacy in this setting.
CONCLUSION
This study illustrates the curative potential of 225Ac-PRIT as a treatment for highly aggressive subtypes of HER2-postive breast cancer. We determined that renal toxicity can be minimized by optimizing relative pharmacologic dosing of the BsAb carrier and radiohapten for safe and effective implementation of 225Ac-PRIT based on HER2 targeting.
DISCLOSURE
This research was funded in part by the Hedvig Hricak Chair in Radiology (to Steven Larson), the Enid A. Haupt Chair (to Nai Kong Cheung), the Center for Targeted Radioimmunotherapy and Theranostics, the Ludwig Center for Cancer Immunotherapy of MSKCC (to Steven Larson), and Mr. William H. Goodwin and Mrs. Alice Goodwin and the Commonwealth Foundation for Cancer Research and the Experimental Therapeutics Center of MSKCC (to Steven Larson and Michael McDevitt). Steven Larson was also supported in part by P50-CA86438. This study also received support from R01-CA233896 (to Sarah Cheal). We also acknowledge P30-CA008748 for use of the Tri-Institutional Laboratory of Comparative Pathology, MSKCC, WCM, and the Rockefeller University, New York, NY; technical services provided by the MSKCC Small-Animal Imaging Core Facility and Laboratory of Comparative Pathology; as well as the Molecular Cytology Core Facility. MSKCC has filed for intraperitoneal protection for inventions related to the α-particle technology of which Michael McDevitt is an inventor. Michael McDevitt was a consultant for Actinium Pharmaceuticals, Regeneron, Progenics, Bridge Medicine, and General Electric. Both MSKCC and Nai Kong Cheung have financial interests in Y-mAbs Therapeutics, Inc., Abpro-Labs, and Lallemand-Biotec Pharmacon. Nai Kong Cheung reports receiving commercial research grants from Y-mAbs Therapeutics, Inc., and Abpro-Labs. Nai Kong Cheung was named as inventor on multiple patents filed by MSKCC, including those licensed to Y-mAbs Therapeutics, Inc., Lallemand-Biotec Pharmacon, and Abpro-Labs. Nai Kong Cheung is a scientific advisory board member for Eureka Therapeutics. Nai Kong Cheung, Steven Larson, and Sarah Cheal were named as inventors in the following patent applications relating to GPA33: SK2014-074, SK2015-091, SK2017-079, SK2018-045, SK2014-116, SK2016-052, and SK2018-068 filed by MSKCC. Steven Larson reports receiving commercial research grants from Genentech, Inc., WILEX AG, Telix Pharmaceuticals Limited, and Regeneron Pharmaceuticals, Inc.; holding ownership interest/equity in Elucida Oncology, Inc., and Y-mAbs Therapeutics, Inc., and holding stock in ImaginAb, Inc. Steven Larson is the inventor and owner of issued patents, both currently unlicensed and licensed by MSKCC to Samus Therapeutics, Inc., Elucida Oncology, Inc., and Y-mAbs Therapeutics, Inc. Steven Larson serves or has served as a consultant to Y-mAbs Therapeutics, Inc., Cynvec LLC, Eli Lilly & Co., Prescient Therapeutics Limited, Advanced Innovative Partners, LLC, Gerson Lehrman Group, Progenics Pharmaceuticals, Inc., and Janssen Pharmaceuticals, Inc. Steven Larson, Nai Kong Cheung, Darren Veach, and Sarah Cheal were named as inventors in PCT/US2021/039418 (THOR cell [tumor homing radio-emitting cell]). Sarah Cheal serves or has served as a consultant to Affibody AB and Primary Insight. No other potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: Can systemic HER2-targeted actinium radiopharmaceutical therapy be curative in mouse models of advanced human breast cancer?
PERTINENT FINDINGS: In preclinical models of advanced human breast cancer, we have shown that treatment with optimized 225Ac-PRIT can result in a high probability of complete responses and durable tumor responses, including histologic cures.
IMPLICATIONS FOR PATIENT CARE: We have identified a targeted α-radiopharmaceutical therapy with a high preclinical therapeutic window for treatment of HER2-expressing advanced breast cancer.
Footnotes
Published online Jun. 5, 2025.
- © 2025 by the Society of Nuclear Medicine and Molecular Imaging.
Immediate Open Access: Creative Commons Attribution 4.0 International License (CC BY) allows users to share and adapt with attribution, excluding materials credited to previous publications. License: https://creativecommons.org/licenses/by/4.0/. Details: https://jnm.snmjournals.org/page/permissions.
REFERENCES
- Received for publication January 25, 2025.
- Accepted for publication May 8, 2025.