Visual Abstract
Abstract
Interleukin-13 receptor α-2 (IL13Rα2) is a cell surface receptor frequently expressed in solid malignancies, such as glioblastoma and melanoma, with limited expression in healthy tissue, rendering it an ideal target for noninvasive and specific tumor delineation. In this study, we report the development of 5 novel IL13Rα2-targeted human monoclonal antibodies (mAbs) KLG-1–5; in subsequent in vitro and in vivo studies after radiolabeling with 89Zr, we evaluate their performance to identify a lead candidate. Methods: Five novel human anti-IL13Rα2 mAbs KLG-1–5 were developed and in vitro binding properties and target specificity assessed. In vivo 89Zr-immuno-PET using KLG-1–5 was conducted in a subcutaneous U-87 MG glioblastoma mouse model, and a mass dose titration study was conducted with lead candidate KLG-3. Ex vivo biodistribution results were used to derive prospective dosimetry of 177Lu-labeled KLG-3. Targeting with KLG-3 was also verified in an A-375 melanoma model using the optimized conditions determined in the U-87 MG xenograft model. Results: In vitro studies confirmed target specificity and pico- to low nanomolar binding affinity. Immuno-PET studies with KLG-1–5 in U-87 MG xenografts demonstrated continuously increasing tumoral uptake with maximal uptake at 144 h after tracer injection, clearance of the unbound tracer from the blood pool, and little uptake in any other normal tissues, leading to high-contrast images. KLG-3 provided the highest tumoral uptake and tumor–to–normal tissue ratios and was chosen as the lead candidate, and further dose optimization with this antibody led to tumoral uptake of 97 ± 6 maximum percent of injected dose per gram at 144 h after tracer injection. Ex vivo biodistribution-derived prospective dosimetry for 177Lu-labeled KLG-3 predicted a favorable therapeutic index, encouraging the development of IL13Rα2-targeted radioimmunotherapy. Of note, KLG-3 performed similarly well in a melanoma model, emphasizing the versatility of this antibody. Conclusion: Lead candidate anti-IL13Rα2 mAb KLG-3 validated highly specific target binding in human glioblastoma and melanoma models, resulting in high-contrast PET images with minimal accumulation in off-target healthy tissues. Prospective dosimetry of its 177Lu-labeled counterpart suggested therapeutic efficacy at relatively low injected activities, supporting further pursuit of KLG-3 in future translational radioimmunotherapy applications.
Interleukin-13 receptor subunit α-2 (IL13Rα2) is a cell membrane–based, high-affinity receptor for interleukin-13 (IL-13) that has garnered much attention as a promising target for cancer therapy because of its increased expression on a variety of malignancies such as glioblastoma, melanoma, and breast cancer (1–3). IL13Rα2 expression is associated with advanced disease and poor prognosis in several of these cancers (4–6). IL13Rα2 was originally thought to be a decoy receptor because of its short cytoplasmic tail; however, more recently, it was found to induce transforming growth factor β production through the activator protein 1 pathway in certain leukemia, pancreatic cancer, ovarian cancer, and glioma cell lines (7–10). Although there is evidence of high expression of this marker in several cancers, its expression in glioblastoma has been studied most rigorously: it is highly expressed in over 80% of glioblastomas, promoting growth, infiltration, and metastasis of glioma cells (11–13).
High expression in several malignancies in conjunction with its lack of expression in healthy brain tissue and limited expression in other healthy tissue (aside from the testes and pituitary gland) renders IL13Rα2 an ideal candidate for use in imaging and potential targeted therapies (14,15). For instance, several IL13Rα2-targeting therapeutic agents, including chimeric antigen receptor T cells based on “IL-13 muteins” with enhanced binding to IL13Rα2 and single-chain fragment variable–based chimeric antigen receptors specific for IL13Rα2 are under investigation (16–20). In contrast, we have been particularly interested in developing antibody-based (radio)therapeutics, aiming to exploit their exquisite target specificity and high binding affinity to achieve high and prolonged tumor uptake and retention, while sparing normal tissues. Here, we report on the generation of anti-IL13Rα2 human monoclonal antibodies (mAbs) using AlivaMab mice (AlivaMab Biologics). Selected Fc-silenced binders were tested in subsequent in vitro and in vivo immuno-PET studies in a mouse model of glioblastoma, yielding a lead candidate, KLG-3, which was further tested in immuno-PET studies in a mouse model of melanoma to assess its potential broader utility. Here, we demonstrate its utility for specific tumor targeting in 2 different tumor-bearing mouse models with sufficient contrast to support its further development for radioimmunotherapy applications.
MATERIALS AND METHODS
Details about the generation of human IL13Rα2-specific mAbs, antibody affinity measurement via biolayer interferometry (BLI), deferoxamine (DFO) conjugation, radiolabeling, in vitro studies, and dosimetry are available in the supplemental materials at http://jnm.snmjournals.org.
Cell Lines
Human glioblastoma cell line U-87 MG (catalog no. HTB-14) and melanoma cell line A-375 (catalog no. CRL-1619) were obtained from American Type Culture Collection. Cell lines were cultured according to American Type Culture Collection recommendations and monitored for mycoplasma contamination using the MycoAlert PLUS Mycoplasma detection kit (Lonza).
Preparation of Human Glioblastoma and Melanoma Xenograft Models in Mice
All animal experiments were performed under an animal use protocol approved by Memorial Sloan Kettering’s Institutional Animal Care and Use Committee according to the American Association for Accreditation of Laboratory Animal Care guidelines. U-87 MG or A-375 cells (4 × 106 in 200 µL) were injected subcutaneously over the right shoulder of 4- to 6-wk-old female athymic nude mice (Charles River). Fourteen days later, the mice underwent intravenous radiotracer administration followed by PET imaging studies.
Small-Animal PET/CT and Biodistribution Studies
For PET imaging studies, tumor-bearing mice were injected intravenously with [89Zr]Zr-DFO-KLG-1–5 (n = 4/group). At the time of radiotracer injection, the tumor size was 200–500 mm3. Serial PET imaging studies were performed at 4, 24, 72, and 144 h (except for immunoglobulin G at a 20-µg mass dose; no scan at 72 h after injection). For blocking studies (n = 4), blocked mice were coinjected with a 10-fold mass excess of unlabeled antibody (KLG-1–5) and imaged 48 h later. In vivo small-animal PET/CT was performed on an Inveon micro-PET/CT instrument (Siemens Manufacturing) (21). Image visualization and volume-of-interest analysis were performed using Amide software, wherein the uptake values for each volume of interest were recorded as mean percent of injected dose ([%ID/g]mean) for normal organs or maximum %ID/g ([%ID/g]max) for tumors. The tumor–to–normal tissue ratio (TNR) was defined as the [%ID/g]max (tumor)/[%ID/g]mean (tissue). Half-lives for biologic clearance from the blood were determined via a monoexponential fit to a volume of interest drawn over the cardiac contents, using Graph Pad Prism software (version 10; GraphPad). Ex vivo biodistribution studies were performed by euthanizing mice at discrete time points after tracer injection. Relevant organs were harvested and weighed, and their radioactivity was determined on an 89Zr-calibrated γ-counter (HidexAMG) using an 890–1,000-keV energy window. Biodistribution values are presented as %ID/g.
Radiation Dosimetry
Ex vivo biodistribution data for the mouse cohorts were used to obtain organ-level radiation-absorbed dose estimates (supplemental materials) (22–26).
Statistics and Data Reporting
All experimental data are presented as mean ± SD. GraphPad Prism 8 or 10 (GraphPad Software, Inc.) was used for statistical analysis. Differences between means were tested by appropriate tests including a t test and ANOVA. The significance level was a P value of less than 0.05.
RESULTS
mAb Production, Selection, Determination of Binding Kinetics, and In Vitro Binding Studies to Target IL13Rα2
Several human mAbs that specifically target IL13Rα2 were developed at the Sanders Tri-TDI by standard hybridoma technology using AlivaMab (Ablexis LLC) transgenic mouse strains (27). After purification from hybridoma supernatants and screening of these for binding to recombinant IL13Rα2 protein and cell binding, the 5 most promising candidates were recombinantly expressed as full-length human immunoglobulin G4, with S228P, F234A, and L235A mutations to attenuate Fcγ receptor interactions (28). Antibodies were conjugated with p-Bz-SCN-DFO by random lysine conjugation in high yields (100% for KLG-1, 98% for KLG-2, 100% for KLG-3, 94% for KLG-4, and 100% for KLG-5). Conjugating the antibodies with DFO did not change the binding characteristics for the recombinant antibodies compared with their unconjugated version, except for KLG-4, in which the dissociation rate constant dropped from 10−5 to 10−3 s−1 (Table 1).
BLI-Determined Binding Characteristics of Unconjugated and DFO-Conjugated Recombinant Antibodies
All DFO-conjugated antibodies were labeled with 89Zr with high radiochemical purity as evident by instant thin-layer chromatography silica gel analysis (Supplemental Fig. 1). Similar to the BLI studies, the antibodies were assessed and characterized by size-exclusion chromatography high-performance liquid chromatography at the initial stage and after DFO conjugation and 89Zr radiolabeling. There was a small amount of antibody dimer or aggregate formation as evident by a small peak at 5.5 min on the high-performance liquid chromatography trace (3.71% for KLG-1, 4.84% for KLG-3, 6.03% for KLG-4, and 4.3% for KLG-5) (Supplemental Fig. 2). All antibody solutions were sterile filtered through a 0.3-µm filter before animal injection to remove aggregate formation, so this aggregate was not present in the in vivo studies. All antibodies were robust and showed identical peaks at each stage of conjugation and radiolabeling, and radiation detection of labeled antibodies by high-performance liquid chromatography was similarly clean. Using matrix-assisted laser desorption ionization time of flight mass spectrometry, antibodies exhibited a degree of DFO conjugation between 0.2 and 1.5 (Supplemental Figs. 3–12; Supplemental Table 1).
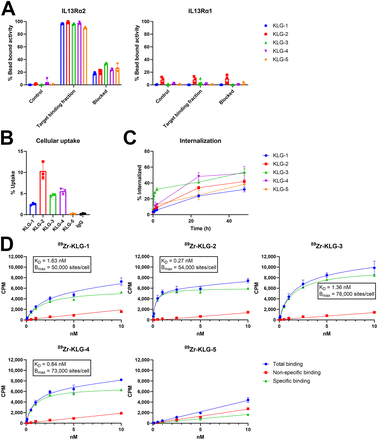
Antigen specificity and binding of radiolabeled antibodies KLG-1–5 was confirmed using bead-based immunoreactivity assays using human IL13Rα1 and IL13Rα2 antigens (29). All antibodies showed excellent binding to the IL13Rα2 target, with very high immunoreactive fractions (96%, 97%, 93%, 96%, and 89% for KLG-1–5, respectively), which was blockable by addition of excess unlabeled antibody (P < 0.0001 for all), demonstrating specificity. Additionally, all antibodies showed little to no off-target binding to IL13Rα1 (0%, 4%, 0%, 3%, and 0% for KLG-1–5, respectively) (Fig. 1A). In vitro cell-based binding, internalization, and saturation binding assays on a glioblastoma cell line expressing IL13Rα2 (U-87 MG) were conducted with all 5 radiolabeled antibodies. IL13Rα2 expression of U-87 MG cells was confirmed by flow cytometry (Supplemental Fig. 13). Antibodies showed various uptake after incubation at 37°C for 1 h (2.5%, 10%, 4.7%, 5.5%, and 0.2% for KLG-1–5, respectively) (Fig. 1B). Surprisingly, KLG-5 showed very little uptake in vitro even though BLI results suggested high affinity to the target antigen. All labeled antibodies demonstrated similar internalization behavior: low over the initial 2 h (5%–12%) but moderate after 1 d (24%–48%) and even higher after 2 d (31%–53%) (Fig. 1C). KLG-3 showed higher internalization at the earlier time points before 24 h (P < 0.0001 for KLG-1, KLG-2, KLG-4, and KLG-5; P value from ANOVA). In saturation binding assays, KLG-1–4 showed nano- to picomolar affinity with expected rectangular hyperbolic curves, whereas KLG-5 showed linear results, consistent with the lack of binding in the cellular uptake data (Fig. 1D).
In vitro characterization of IL13Rα2-targeting mAbs with human IL13Rα2 and IL13Rα1 antigens and U-87 MG. (A) Immunoreactivity assay. (B) Uptake assay. (C) Internalization. (D) Saturation binding with U-87 MG. Bmax = maximum number of binding sites; CPM = counts per minute; IgG = immunoglobulin G; KD = equilibrium dissociation constant.
In a competition binding assay between the recombinant human IL-13 cytokine and a representative antibody (KLG-3), KLG-3 was able to displace bound human IL-13 on U-87 MG cells with an inhibitory concentration of 50% of 0.42 nM (Supplemental Fig. 14); that is, IL-13 does not inhibit antibody binding until it is present in large excess and IL-13 and KLG-3 occupy the same binding site on the cellular receptor.
In Vivo PET Imaging Studies in a Subcutaneous U-87 MG Xenograft Mouse Model
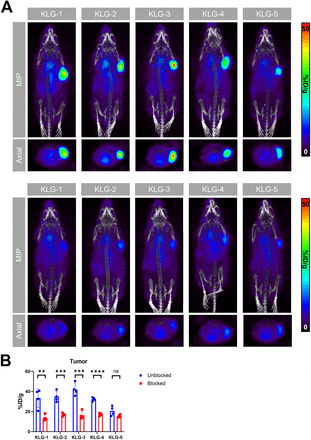
In serial imaging studies after the intravenous administration of 89Zr-labeled KLG-1–5 (20 µg/mouse), all antibodies showed continuously increasing tumor uptake, with maximum uptake at 144 h ([%ID/g]max, 47 ± 12 for KLG-1, 35 ± 11 for KLG-2, 71 ± 4 for KLG-3, 42 ± 5 for KLG-4, and 53 ± 9 for KLG-5) (Figs. 2A and 2B). Uptake in the immunoglobulin G control plateaued after 24 h and at 144 h after injection ([%ID/g]max, 22 ± 5) was significantly lower than with the targeting antibodies (Fig. 2C), except for KLG-2. Tumor uptake was coupled with consistent slow clearance of unbound antibodies from the blood pool with terminal blood half-lives of 121 ± 35, 76 ± 21, 96 ± 19, 100 ± 30, 125 ± 47, and 99 ± 14 h for KLG-1–5 and immunoglobulin G control, respectively (Fig. 2D; Supplemental Table 2), and minimal uptake in any normal tissues, leading to high-contrast images with TNRs for blood, kidneys, and muscle of 6.2–8.7, 11–16, and 23–42, respectively. The blood TNR for all targeting antibodies was significantly higher than that of the isotype control at 144 h (Fig. 2E; Supplemental Table 3). Overall, KLG-3 showed the highest tumor uptake and TNRs.
In vivo serial imaging of IL13Rα2-targeting mAbs and isotype control in U-87 MG xenograft model. Imaging was conducted at 4, 24, 72, and 144 h after injection (except for immunoglobulin G [IgG]; no scan at 72 h after injection). (A) Representative maximum-intensity projection (MIP) and axial PET/CT images of nude mice bearing U-87 MG xenografts injected with 20 µg (3.7 MBq) of 89Zr-labeled mAbs. (B) Tumoral uptake ([%ID/g]max). (C) Comparison of tumor uptake ([%ID/g]max). (D) Blood clearance ([%ID/g]mean) with exponential fit to determine blood half-life. (E) Comparison of blood TNRs (TNR-B). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001; P values shown from ANOVA. ns = not significant.
To confirm the target specificity and lack of nonspecific binding in vivo, blocking studies were conducted. The unblocked cohort again showed high tumoral uptake for all antibodies similar to prior imaging study ([%ID/g]max, 33% ± 9% for KLG-1, 35% ± 5% for KLG-2, 42% ± 7% for KLG-3, 32% ± 2% for KLG-4, and 21% ± 5% for KLG-5), which was substantially lower in the blocked cohort ([%ID/g]max, 14% ± 3% for KLG-1, 17% ± 2% for KLG-2, 16% ± 4% for KLG-3, 17% ± 1% for KLG-4, and 15% ± 1% for KLG-5) (Figs. 3A and 3B).
In vivo blocking study in U-87 MG xenograft model. (A) Representative maximum-intensity projection (MIP) and axial PET/CT images after injection with 20 µg (3.7 MBq) of 89Zr-labeled KLG-1–5 without (unblocked, top) or with (blocked, bottom) coinjection of 200 µg of unlabeled antibody. Imaging was conducted at 48 h after injection. (B) Comparison of tumoral uptake ([%ID/g]max) of blocked and unblocked cohorts (n = 4). **P < 0.01, ***P < 0.001, ****P < 0.0001; P values are from t test. ns = not significant.
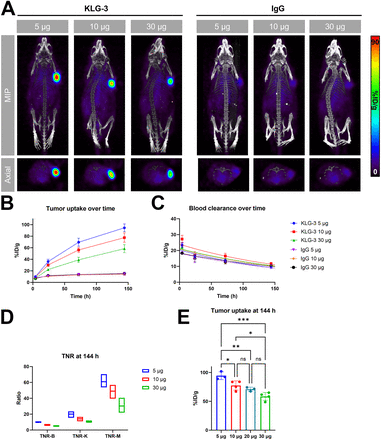
Based on these in vitro and in vivo studies, KLG-3 was determined to be the most promising candidate and was used in a dose optimization study to investigate the influence of the injected mass on the biodistribution. Again, for each mass dose, the maximum tumoral uptake was seen at 144 h after injection. Tumor uptake decreased with higher antibody mass ([%ID/g]max, 97 ± 6 for 5 µg, 77 ± 8 for 10 µg, and 58 ± 7 for 30 µg) (Figs. 4A and 4B). Terminal blood clearance was not substantially affected by changing administered mass (Fig. 4C; Supplemental Table 4). High tumoral uptake at lower mass doses translated to high TNRs for the 5-µg dose (10, 20, and 60 for blood, kidneys, and muscle, respectively; Fig. 4D). Including the already obtained data with the 20-µg mass dose, this shows a continuously decreasing tumoral uptake with increasing antibody mass dose (Fig. 4E).
In vivo imaging of U-87 MG xenograft model with varying mass doses of 89Zr-labeled KLG-3 or isotype control. (A) Representative maximum-intensity projection (MIP) and axial PET/CT images at 144 h after injection. (B) Tumoral uptake ([%ID/g]max). (C) Blood clearance ([%ID/g]mean). (D) TNR of 5, 10, and 30 µg at 144 h after injection. (E) Comparison of tumoral uptake ([%ID/g]max) at 144 h after injection of all masses (5, 10, 20, and 30 µg) of 89Zr-labeled KLG-3. *P < 0.05, **P < 0.01, ***P < 0.001; P values shown from ANOVA. IgG = immunoglobulin G; ns = not significant; TNR-B = blood TNR; TNR-K = kidney TNR; TNR-M = muscle TNR.
Ex Vivo Biodistribution and Prospective Dosimetry Support Radioimmunotherapy with 177Lu-Labeled KLG-3
Monte Carlo radiation dose modeling, based on ex vivo biodistribution data with 89Zr-labeled KLG-3 (Supplemental Fig. 15), for the 177Lu-labeled counterpart showed that the tumor tissue received the highest dose of 7.32 Gy/MBq (Table 2) and in conjunction with the observed therapeutic indices support the use of KLG-3 in future preclinical radioimmunotherapy studies. Of note, the dose to the tumor is likely underestimated in this model as the maximum tumor uptake was not yet reached at the latest biodistribution time point.
Monte Carlo Radiation Dose Modeling Prospective Dosimetry to Tumor and normal Tissue with 177Lu-Labeled KLG-3
Melanoma Model Validates Target Binding In Vitro and In Vivo
Expression of IL13Rα2 in human melanoma cells (A-375), assessed by flow cytometry (Supplemental Fig. 13), was higher than in the U-87 MG cell line. Further studies were confined to our lead candidate mAb KLG-3. Cellular internalization of KLG-3 in the A-375 cells was faster and higher than in the U-87 MG cells (P < 0.0001), whereas similar results were obtained in the saturation binding assay (Supplemental Figs. 16A and 16B).
Serial imaging studies in a subcutaneous A-375 xenograft mouse model, using a 5-µg mass dose, revealed increasing tumoral uptake over time, with a maximum at the latest 144-h time point after injection. Although tumor uptake at 144 h was lower ([%ID/g]max, 51 ± 5) than that in the U-87 MG model ([%ID/g]max, 97 ± 6), it was still significantly higher than that in the isotype control ([%ID/g]max, 8.0 ± 2.5; P < 0.0001) (Supplemental Figs. 16C and 16D). Similar to the findings in the U-87 MG model, KLG-3 uptake was low in any other tissues, with TNRs of 4.5 ± 0.7, 11.2 ± 0.9, and 44 ± 5.5 for blood, kidneys, and muscle, respectively. TNRs for the isotype control antibody were significantly lower (1.9 ± 0.9, 5.2 ± 3.9, and 10 ± 3.8; P ≤ 0.004 for all) (Supplemental Fig. 16E).
DISCUSSION
We successfully developed a set of human IL13Rα2-targeting mAbs, verified their target specificity, assessed binding kinetics, and identified the lead candidate KLG-3. 89Zr-immuno-PET studies in subcutaneous glioblastoma and melanoma mouse models showed continuously increasing, high tumor uptake and high TNRs. These results, along with encouraging therapeutic dosimetry estimates for the 177Lu-labeled counterpart, support further translational development of an anti-IL13Rα2 theranostic platform.
In vitro assays conducted on the glioblastoma cell line U-87 MG showed moderate expression levels of IL13Rα2. Cellular uptake with the U-87 MG cells with all selected antibodies showed a range of uptake (KLG-2 > KLG-4 > KLG-3 > KLG-1 > KLG-5), which correlated well to the association rate constants (determined by BLI), with KLG-2 binding fastest, KLG-5 binding considerably slower, and the remaining 3 antibodies demonstrating similar binding rates. This ranking was retained in saturation binding assays. When cells were incubated for 1 h, the saturation binding assay showed linear results for KLG-5. This aligns with the similarly low cellular uptake at the 1-h incubation period and the slow binding rate. These data also suggest that cell-based saturation binding assay determination of the equilibrium dissociation constant of an antibody is subject to interactions and influences (avidity) outside of the biochemical antibody–antigen binding interaction (affinity) achieved by BLI. An internalization assay conducted over 48 h in U-87 MG cells yielded similar overall uptake at the latest time point: KLG-3 and KLG-4 showed the highest degree of internalization, but internalization was faster for KLG-3 than for the other antibodies. This internalization could offer advantages in therapeutic applications, especially with short pathlength radiation (e.g., α- or Auger electrons).
Initial in vivo PET imaging studies at a mass dose of 20 µg of all antibodies (including the isotype control) showed similar clearance over the 144-h imaging time period with minimal uptake or accumulation in off-target tissue and continued blood clearance. The lack of accumulation in the liver and kidneys (even after 144 h) is likely due to the Fc silencing and Fab′ arm exchange mutations of the antibodies, respectively (30). Of note, our antibodies are not cross-reactive to the murine antigen, hence the lack of binding to endogenous murine IL13Rα2 in these models. These factors appeared to have increased the in vivo half-life of these antibodies, resulting in moderate blood activity after 144 h and enabling increasing tumoral uptake over time, as the antibody remained in circulation. The lack of accumulation in bone or lymph nodes for any of the antibodies proves their robustness in vivo and the lack of 89Zr deincorporation from the conjugate (31). The high tumoral uptake of all targeting antibodies, significantly higher than with the isotype control, further demonstrates specificity in vivo. KLG-3 demonstrated the highest tumoral uptake and TNRs and was thus selected as the lead candidate.
Interestingly, lower amounts of unlabeled KLG-3 resulted in markedly higher tumor uptake and contrast, with the lowest mass dose (5 µg) leading to the highest uptake, highlighting the importance of dose titration for the individual target antigen. That is, higher antibody mass doses exert a blocking effect leading to receptor saturation and lower tumoral uptake. Encouragingly, Monte Carlo dosimetry estimates in the U-87 MG model suggested that for 177Lu-labeled KLG-3 tumoricidal absorbed doses will be achievable with tolerable radiation burden for at-risk organs. In fact, the dose to the tumor was likely underestimated in these calculations since the tumoral uptake of the antibody did not plateau over the time period studied. Overall, the therapeutic indices support further studies evaluating its therapeutic potential.
To demonstrate the versatility of our newly developed antibodies, we pursued additional studies with the lead candidate KLG-3 using the human melanoma cell line A-375. KLG-3 exhibited binding affinity similar to that in the U-87 MG model, but the sites per cell of A-375 cells were higher compared with the U-87 MG cells. Surprisingly, whereas in vivo PET imaging showed continuously increasing tumoral uptake, similarly to the U-87 MG model, total tumoral uptake was lower than that in the U-87 MG model at the same mass dose. Tumoral uptake of the isotype control in each model also varied, with higher uptake in the U-87 MG model (15%) compared with the A-375 model (8%) at 144 h. This is possibly due to differences in blood flow into the tumor and by differences in vascularity and the tumor microenvironment between melanoma and glioblastoma xenografts, although more studies are needed. Nevertheless, even with the lower tumoral uptake compared with the U-87 MG model, high TNRs were achieved in the melanoma model, leading to high-contrast images with [89Zr]Zr-DFO-KLG-3 and demonstrating its strength in more than 1 model.
So far, the reported radiolabeled IL13Rα2-targeting antibodies include IL13Rα2-AB08-v1010-hG1 that was assessed after DFO conjugation and labeling with 89Zr in a subcutaneous melanoma mouse model (A-375 cells) (32). Maximum tumoral uptake by PET imaging was noted at 96 h after injection (19.84 %ID/g) at an antibody mass dose of 60 µg and blood activity of 7.47 %ID/g, with tumoral uptake and blood activity decreasing at later time points. Ex vivo biodistribution showed tumor accumulation of only 10.05 %ID/g at 24 h and 8.5 %ID/g at 264 h. This contrasts with our antibody, which showed increasing tumoral uptake, reaching a maximum at 144 h with 97 %ID/gmax at a 5-µg mass dose of antibody. In addition, an IL13Rα2-targeting peptide, Pep-1L, was labeled with 64Cu and has demonstrated tumoral uptake of 2.43 ± 0.626 %ID/g at 24 h after injection in a subcutaneous glioblastoma model and of 14.09 ± 0.246 %ID/g at 4 h after injection in an IL13Rα2-inducible melanoma model compared with noninduced tumor-bearing mice (4.179 ± 0.007 %ID/g) (33). Furthermore, efficacy on convection-enhanced delivery of 225Ac-labeled Pep-1L in an orthotopic glioblastoma model was reported (34). A recent study using [99mTc]Tc-MAG3-Pep-1 reported an equilibrium dissociation constant of 78.13 ± 11.52 nM, which is markedly higher than that of our newly developed antibodies (35). Therefore, we believe that our lead candidate KLG-3 has distinct advantages over the aforementioned radiolabeled IL13Rα2-targeting moieties.
In summary, lead candidate KLG-3 met several requirements for a successful radioimmunotherapy drug, including specific, high affinity target binding, slow dissociation, and fast internalization. Paired with the lack of accumulation in the spleen, kidneys, or liver (supported by specific mutations attenuating Fcγ receptor interactions), this allowed for high antibody accumulation in the tumor. Lastly, IL13Rα2 was found to be highly expressed on tumor cells with reportedly minimal expression in healthy tissues, thus ensuring minimal toxicity.
CONCLUSION
Lead candidate anti-IL13Rα2 mAb KLG-3 showed highly specific target binding in human glioblastoma and melanoma models, resulting in high-contrast PET images with minimal accumulation in off-target healthy tissues. Prospective dosimetry of its 177Lu-labeled counterpart suggests therapeutic efficacy at relatively low injected activities, supporting its use in future translational radioimmunotherapy applications.
DISCLOSURE
Memorial Sloan Kettering has filed for patent protection on behalf of Leah Gajecki, Irina Lebedeva, David Andrew, Manuel Baca, Darren Veach, and Simone Krebs for inventions related to the work described in this paper. Simone Krebs is supported by NIH R37 CA262557, Mr. William H. Goodwin and Mrs. Alice Goodwin and the Commonwealth Foundation for Cancer Research, and The Center for Experimental Therapeutics of Memorial Sloan Kettering Cancer Center, flexTDF Award, and TDF Award. Jason Lewis acknowledges support from NIH R35 CA232130. This work was also supported by NIH/NCI Cancer Center Support Grant P30 CA008748. Simone Krebs has consulted for Telix Pharmaceuticals Ltd. Steven Larson reports receiving commercial research grants from Y-mAbs Therapeutics, Genentech, Inc., WILEX AG, Telix Pharmaceuticals Limited, and Regeneron Pharmaceuticals, Inc.; holding ownership interest/equity in Voreyda Theranostics Inc. and Elucida Oncology Inc., and holding stock in Y-mAbs Therapeutics. Steven Larson is the inventor and owner of issued patents both currently unlicensed and licensed by Memorial Sloan Kettering Samus Therapeutics, Inc., Y-mAbs Therapeutics Inc., and Elucida Oncology, Inc.; is or has served as a consultant to Cynvec LLC, Eli Lilly & Co., Prescient Therapeutics Limited, Advanced Innovative Partners, LLC, Gerson Lehrman Group, Progenics Pharmaceuticals, Inc., and Janssen Pharmaceuticals, Inc. Luka Carter has consulted for Evergreen Theranostics. Michael Postow reports support from Bristol Myers Squibb, Infinity, Merck, Novartis, and RGenix, and has consulted for Bristol Meyer Squibb, Chugai, Erasca, Eisai, Merck, Nektar Therapeutics, Novartis, Pfizer, and Replimune. No other potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: Out of 5 novel IL13Rα2-targeted human monoclonal antibodies (KLG-1–5), can a lead candidate be identified as suitable for delineating malignancies in 89Zr-immuno-PET studies?
PERTINENT FINDINGS: Immuno-PET studies with KLG-1–5 in subcutaneous glioblastoma xenografts demonstrated continuously increasing tumoral uptake, with maximal uptake at day 6 after tracer injection, clearance of the unbound tracer from the blood pool, and little uptake in any other normal tissues, leading to very high contrast images. KLG-3 provided the highest tumoral uptake and TNRs and was chosen as the lead candidate, and further dose optimization with this antibody led to tumoral uptake of 97 ± 6 [%ID/g]max at day 6 after tracer injection. KLG-3 performed similarly well in a melanoma model, showcasing the versatility of this antibody.
IMPLICATIONS FOR PATIENT CARE: Lead candidate KLG-3 validated highly specific target binding in tumor-bearing models, resulting in high-contrast PET images with minimal accumulation in off-target healthy tissues with the potential for translational radioimmunotherapy applications.
ACKNOWLEDGMENTS
We gratefully acknowledge the staff members of the Small-Animal Imaging Core (Valerie Longo and Pat Zanzonico) and Leah Bassity for editing assistance.
Footnotes
Published online Feb. 20, 2025.
- © 2025 by the Society of Nuclear Medicine and Molecular Imaging.
REFERENCES
- Received for publication September 7, 2024.
- Accepted for publication January 15, 2025.