Abstract
Fibrosing lung diseases affect over 160,000 individuals in the United States alone and can carry a prognosis that is worse than many cancers. Antifibrotic treatments modify only the rate of fibrosis progression, and more effective therapies are urgently needed. Molecular imaging enables visualization of disease pathogenesis in progress. It provides a noninvasive means to monitor and quantify dysregulated molecular fibrotic pathways and shows great promise in aiding the diagnosis and disease activity monitoring of pulmonary fibrosis. Here, we review molecular imaging probes under development for use in pulmonary fibrosis. We provide our opinion on current challenges in translating preclinical molecular imaging probes into clinical successes, as well as future directions for expanding their use in drug development.
Interstitial lung diseases (ILDs) are a diverse group of pulmonary pathologies represented by varying degrees of inflammation or fibrosis. The prototypic progressive fibrosing ILD is idiopathic pulmonary fibrosis (IPF). IPF carries a high morbidity and mortality. Average life expectancy from the time of diagnosis is less than 4 y by some estimates, which is worse than many cancers (1).
Many other non-IPF ILDs such as those associated with systemic sclerosis and other autoimmune conditions will display a progressive fibrosing course that is similar to IPF (2). Although the disease course of IPF is overall progressive, some patients exhibit rapidly worsening disease, some have a more indolent course, and still others may experience periods of stability interspersed with acute exacerbations, which are often life-threatening. Currently, there is no single-time-point measurement of fibrotic disease activity. Pulmonary function testing and high-resolution CT, which allows for visualization of increased extracellular matrix deposition in the form of honeycomb cysts, architectural distortion, and reticulation, are the mainstay diagnostics to monitor disease progression, but each provides limited information on the rate at which progression occurs unless performed serially.
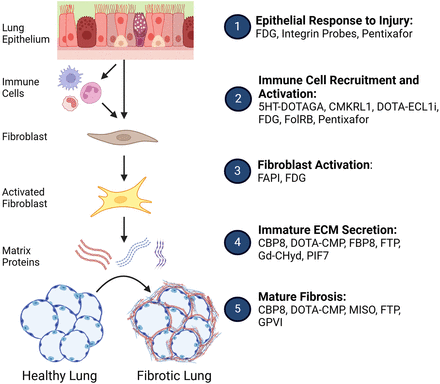
Efforts to improve imaging of the fibrotic lung have included developing molecular probes to target areas of fibrosis or fibrotic activity with greater specificity to complement the structural information afforded by high-resolution CT. The goal of this short review is to introduce the reader to the breadth of molecular imaging targets (Fig. 1) that are being tested in pulmonary fibrosis, as well as to discuss challenges and future directions for the field.
Phases of pulmonary fibrosis pathogenesis and relevant molecular probes. CMKRL1 = chemerin chemokine-like receptor 1; ECM = extracellular matrix; FolRB = folate-receptor-β; FTP = fibronectin-targeting peptide; GPVI = platelet-glycoprotein VI; MISO = fluoromisonidazole. Created in BioRender. Allison, M. (2025) https://BioRender.com/x60q386.
MOLECULAR MRI OF PULMONARY FIBROSIS
Type I collagen is a major component of the fibrotic extracellular matrix. Molecular MRI with a collagen-specific peptide conjugated to 3 Gd3+ chelates specifically detected and quantified lung fibrosis in a mouse bleomycin injury model (3). A similar result was found using a different collagen-targeting peptide conjugated to a Gd3+-binding protein for signal enhancement (4). During active fibrogenesis, enzymes convert lysine side chains on collagens to allysines (5). An allysine-binding Gd3+ chelate quantified fibrogenesis in the bleomycin mouse model and distinguished new fibrosis from stable scarring, with an improved probe, Gd-CHyd, showing an 80% increase in lung signal enhancement compared with the original probe (6,7). Mn2+-based allysine probes had a 3-fold increase in relaxivity when bound to collagen allysine compared with their unbound state, and precise regional quantification of fibrogenesis was confirmed by ex vivo histologic correlation (8). Molecular MR probes can also be sensitive to enzymatic activity. Gd-5-HT-DOTAGA is oligomerized in the presence of the inflammatory enzyme myeloperoxidase, and oligomerization resulted in increased relaxivity and retention in inflamed tissue when tested in bleomycin-injured mice (9). Whether these probes can identify active disease in clinical populations remains to be seen.
PET IMAGING IN PULMONARY FIBROSIS
18F-FDG is the most studied PET probe in IPF. It is purported to detect the increased metabolic activity of inflammatory cells and activated fibroblasts, although it cannot distinguish between the metabolic activity of cells participating in normal healing from those participating in an abnormal fibrotic response. In both IPF and systemic sclerosis–associated ILD, 18F-FDG uptake was increased in patients compared with healthy controls, and increased uptake was correlated with disease severity within the affected groups (10–12). It was only mildly successful at independently predicting disease progression, however, requiring multivariate analysis using more conventional disease markers to strengthen these predictions (13). 18F-FDG PET was also unable to detect a treatment response in IPF patients on antifibrotic therapies (14). A study of IPF patients undergoing thoracic surgery determined that 18F-FDG uptake was higher in those patients who developed postsurgical acute exacerbations than in patients who did not (15). Together, these results suggest that 18F-FDG PET may provide insights into acute disease activity but may be less helpful for predicting disease progression or treatment response.
Extracellular matrix proteins are a popular target for PET probe development. 68Ga-DOTA–CMP is a conjugated collagen-mimetic peptide that binds to damaged collagen fibrils, and uptake was increased in lungs of bleomycin-injured mice compared with control mice (16). Type 1 collagen has also been directly targeted using a peptide probe (CBP8) labeled with either 64Cu or 68Ga; lung uptake linearly correlated with collagen content quantified biochemically in 2 different mouse models and was also able to detect treatment response (17,18). 68Ga-CBP8 was tested in 5 healthy volunteers and 9 IPF patients and was found to preferentially accumulate in fibrotic areas identified on high-resolution CT but was also seen in regions where the CT was normal, suggesting increased sensitivity for active fibrosis (Fig. 2) (19,20). 68Ga-CBP8 was also able to detect decreases in collagen in response to treatment with a dual integrin inhibitor, bexotegrast, in a randomized controlled trial of IPF patients (21). These results support that 68Ga-CBP8 could be used to detect changes in response to antifibrotic therapy. Platelet-glycoprotein VI is expressed on the surface of platelets and binds to multiple components of the extracellular matrix, including both collagen and fibronectin; 64Cu-glycoprotein VI showed increased uptake in the lungs of bleomycin-injured mice, with peak uptake occurring at the time point of maximal Ashcroft scores of fibrosis severity (22). Fibronectin alone was detectable at early time points in bleomycin-injured mice using a 64Cu-labeled fibronectin-targeting peptide, suggesting that it could be an early marker of fibrosis (23). Similarly to MRI, allysine-targeted PET probes, including 68Ga-PIF7, showed uptake that linearly correlated with lung allysine content (24,25).
68Ga-CBP8 uptake in healthy subject (A) and IPF subject (B and C) showing uptake not only in areas of established fibrosis (red arrows) but also in regions without visible fibrotic changes on CT (white arrows). (Adapted with permission of American Thoracic Society. Copyright © 2024 American Thoracic Society. All rights reserved (19). American Journal of Respiratory and Critical Care Medicine is official journal of American Thoracic Society. Readers are encouraged to read entire article for correct context at https://www.atsjournals.org/doi/full/10.1164/rccm.201903-0503LE. Authors, editors, and American Thoracic Society are not responsible for errors or omissions in adaptations.)
Before the production of collagen and other extracellular matrix proteins, fibroblasts must be activated. Fibroblast activation protein is an endopeptidase expressed on the cell surface of activated fibroblasts (26). Several fibroblast activation protein inhibitors (FAPI) have been repurposed to detect fibroblast activation protein expression. One study examined 68Ga-FAPI uptake in subjects with pulmonary fibrosis who had undergone FAPI scanning to assist with lung cancer diagnosis (27). Fibrotic areas of the lung had a unique uptake signal compared with suspected lung cancer, and subsequent histologic staining of biopsy specimens showed fibroblast activation protein expression at the border of fibrotic and healthy tissue, suggesting specificity of this probe for early fibrosis. The 68Ga-FAPI-04 probe was tested in a pilot study of patients with systemic sclerosis-associated ILD, and uptake was associated with disease progression independently of baseline CT findings or pulmonary function testing; 68Ga-FAPI-04 also detected changes in response to antifibrotic therapy (28). Together, these data suggest that FAPI probes may be useful for disease course prognostication and assessment of treatment response in pulmonary fibrosis.
Integrins are another target of interest in pulmonary fibrosis. Integrins are a family of cell surface adhesion receptors whose expression is upregulated by epithelial injury, which is thought to underlie the development of IPF (26). Several integrin-targeted PET probes are under clinical investigation. One study used an 18F-cysteine-knot peptide to engage integrin αvβ6, whereas another leveraged the integrin-binding envelope protein of the foot-and-mouth disease virus to develop a different αvβ6-specific PET probe (29,30). Both probes accumulated in the fibrotic areas of the lungs of both IPF and ILD patients, with uptake being highest in the most severely affected areas as determined by high-resolution CT. In preclinical testing, uptake of an αvβ3 probe correlated well with histopathologic measurements of lung fibrosis and integrin expression in bleomycin-injured rats (31). Whether these integrin probes prove valuable for prediction of disease progression or treatment response will require further clinical investigation.
Although not specific for the fibrotic process, imaging of pulmonary inflammation (a presumed precursor to fibrosis) is also being pursued. Chemerin chemokine-like receptor 1 is transiently expressed in pulmonary macrophages of IPF patients, and chemerin chemokine-like receptor 1 PET was able to detect inflammation in bleomycin-injured mice; uptake correlated with subsequent increases in fibrosis (32). Proinflammatory macrophages express folate-receptor-β; an 18F-folate probe demonstrated that folate-receptor-β is increased in human patients with fibrotic ILD and that folate-receptor-β expression increased in parallel with fibrosis score severity (33). C-C chemokine-receptor 2–positive monocytes are increased in the lungs of patients with IPF and mediate fibroblast recruitment and collagen production (34). The C-C chemokine-receptor 2–targeted PET tracer 64Cu-DOTA-ECL1i was trialed in both bleomycin-injured mice and human subjects with IPF (35). Probe uptake in mice was highest 2 wk after the start of bleomycin treatment and remained elevated several weeks after bleomycin treatment, suggesting the detection of a fibrotic response. In a small number of patients with IPF, uptake was increased compared with healthy controls and was most enhanced in regions of reticulation and honeycombing seen on CT. Hypoxia-related genes have been found to be upregulated in the lungs of IPF patients, and preclinical data suggested that 18F-fluoromisonidazole uptake, a marker of hypoxia, may be indicative of progressive fibrosis (36). The 18F-fluoromisonidazole probe was unable to detect increased uptake in IPF patients, however, suggesting that functional hypoxia may have less of a role in IPF pathogenesis than initially hypothesized (37). Lastly, a fibrin-targeted PET probe, 64Cu-FBP8, demonstrated increased signal in the lung of IPF patients compared with controls, a finding that built on earlier mouse studies supporting ongoing lung injury as a prominent feature of IPF (38).
CHALLENGES AND FUTURE DIRECTIONS
The physiology of a healthy lung presents unique challenges to molecular imaging. Blood and air volumes are higher than in other organs and change with each breath. Correction factors have been proposed but are not yet standardized in the field (39,40). These issues are compounded in diseases that alter tissue density and perfusion, leading to changes in the relative fractions of air, blood, and lung tissue (41). For PET, both motion and changes in tissue density with disease will also impact the accuracy of the attenuation correction. Most of the studies cited here have reported only static imaging; only a few have reported kinetic modeling. Even the accuracy of modeling studies will depend on addressing these issues. Many of the papers included in this review do not address these confounders to their results.
Another major challenge is accounting for the heterogeneity of pulmonary fibrosis; no consensus exists on how to appropriately analyze the imaging data generated. Is the magnitude of probe uptake the more relevant measure, or is it the extent of the lung that takes up the probe? Some groups report data based on the whole lung, others based on a defined affected area; some correct for tissue density, others do not. Various measurements including SUVmax, SUVmean, and tissue-to-background ratios have been used, but none consistently (19,42,43). Furthermore, in the absence of a consistent output measure, it is impossible to compare the performance of different probes across studies either in preclinical or clinical testing. Ultimately, clinical application demands a clear, measurable output that can be easily and widely implemented; a requirement for complex modeling for data interpretation would be beyond the scope of most clinical workflows.
The characteristics of specific nuclides (such as 18F, 68Ga, and 64Cu) used for radiolabeling the molecules discussed in this review may influence the interpretation of signals in the lung. For example, 68Ga emits positrons with a higher maximum energy (1.899 MeV) than that of 18F (0.635 MeV), resulting in a greater positron range (44). This is an intrinsic factor that affects the spatial resolution of PET imaging. However, when whole-body PET scanners are used, the impact of the higher positron range on the spatial resolution of the final image is not a significant concern, particularly if respiratory motion is not compensated for. Additionally, 64Cu has a substantially longer half-life (12.7 h) than that of 18F (109.7 min) and 68Ga (68 min). This extended half-life enhances the capability of 64Cu-labeled radiopharmaceuticals to conduct delayed imaging at times when background signals may be significantly reduced, provided that free 64Cu or radiolabeled metabolites do not accumulate either in the lung or in adjacent organs (e.g., liver). Radiolabeled metabolites in the exhaled air would be particularly problematic for lung imaging, although this has not been reported for any of the radiopharmaceuticals discussed here.
On the preclinical side, the use of the bleomycin mouse model is less than ideal; rodents can heal after bleomycin-induced lung injury in a way that patients with IPF do not (45). Thus, it is difficult to use this model to test probes that cannot distinguish normal wound healing from the abnormal fibrotic response, possibly explaining why certain probes are less successful in predicting the disease course in humans. Developing better animal models of pulmonary fibrosis will be essential to continue to advance the field of molecular imaging for human applications.
One area in which molecular imaging already plays a clear role is for confirming target engagement and evaluating treatment response (46). Changes in C-X-C chemokine receptor type 4 expression as detected by uptake of 68Ga-pentixafor were able to predict responses to antifibrotic therapy, as was the FAPI probe discussed earlier (28,47). The use of an αvβ6-targeted PET probe showed that an inhaled αvβ6 inhibitor was able to reach the lungs and engage the intended receptors, resulting in their internalization and degradation (48). A different αvβ6-targeted probe was used to confirm target engagement for bexotegrast, an integrin inhibitor under investigation for use in IPF treatment (49). As understanding of the molecular underpinnings of IPF continues to expand, more targeted therapies may be developed, and molecular imaging of those same targets may become essential in confirming appropriate drug delivery and action during the early stages of development.
CONCLUSION
By enabling direct imaging of aberrant fibrotic pathways, molecular imaging both offers a unique approach to enhance our understanding of pathophysiology and holds the potential for improved disease activity detection and monitoring. Yet there are no molecular imaging probes with clinical indications for pulmonary fibrosis. Clinical studies are often biased to using probes that are widely available, such as 18F-FDG and now the FAPI probes. Novel probes are typically reported in only single-center proof-of-concept studies. The much higher mass dose of the MRI probes necessitates expensive safety and pharmacology studies before first-in-human evaluations, and the cost of these studies is too high for academic researchers. The clinical need for effective molecular imaging in pulmonary fibrosis is high. Current antifibrotic therapies are expensive and beneficial to only a subset of patients. An imaging test that could identify an effective antifibrotic therapy would be commercially viable, and we hope that this unmet need acts as a pull for industry to commercialize one or more of these PET or MRI probe technologies to accelerate the development of new therapies for a devastating disease.
DISCLOSURE
This work is supported by NHLBI T32HL116275 to Margaret Allison, K23HL150331 and R01HL171240 to Sydney Montesi, K25HL148837 to Iris Zhou, and R01HL153606 to Peter Caravan and Ciprian Catana. Peter Caravan reports research funding from Pliant Therapeutics, Transcode Therapeutics, and Canon Medical, as well as consulting fees from and equity in Collagen Medical LLC and Reveal Pharmaceuticals. Sydney Montesi reports research funding from Pliant Therapeutics and Boehringer Ingelheim; consulting fees from Accendatech USA, DevPro Biopharma, Gilead Sciences, Mediar Therapeutics, and Roche; advisory board fees from APIE Therapeutics and Pliant Therapeutics; speaking fees from Cowen; and royalties from Wolters Kluwer. No other potential conflict of interest relevant to this article was reported.
Footnotes
Published online Feb. 27, 2025.
- © 2025 by the Society of Nuclear Medicine and Molecular Imaging.
REFERENCES
- Received for publication October 31, 2024.
- Accepted for publication February 3, 2025.