Visual Abstract
Abstract
The epidermal growth factor receptor (EGFR) protein is highly expressed in a range of malignancies. Although therapeutic interventions directed toward EGFR have yielded therapeutic responses in cancer patients, side effects are common because of normal-tissue expression of wild-type EGFR. We developed a novel tumor-specific anti-EGFR chimeric antibody ch806 labeled with 225Ac and evaluated its in vitro properties and therapeutic efficacy in murine models of glioblastoma and colorectal cancer. Methods: 225Ac-ch806 was prepared using different chelators, yielding [225Ac]Ac-macropa-tzPEG3Sq-ch806 and [225Ac]Ac-DOTA-dhPzPEG4-ch806. Radiochemical yield, purity, apparent specific activity, and serum stability of 225Ac-ch806 were quantified. In vitro cell killing effect was examined. The biodistribution and therapeutic efficacy of 225Ac-ch806 were investigated in mice with U87MG.de2-7 and DiFi tumors. Pharmacodynamic analysis of tumors after therapy was performed, including DNA double-strand break immunofluorescence of γH2AX, as well as immunohistochemistry for proliferation, cell cycle arrest, and apoptosis. Results: [225Ac]Ac-macropa-tzPEG3Sq-ch806 surpassed [225Ac]Ac-DOTA-dhPzPEG4-ch806 in radiochemical yield, purity, apparent specific activity, and serum stability. [225Ac]Ac-macropa-tzPEG3Sq-ch806 was therefore used for both in vitro and in vivo studies. It displayed a significant, specific, and dose-dependent in vitro cell-killing effect in U87MG.de2-7 cells. 225Ac-ch806 also displayed high tumor uptake and minimal uptake in normal tissues. 225Ac-ch806 significantly inhibited tumor growth and prolonged survival in both U87MG.de2-7 and DiFi models. Enhanced γH2AX staining was observed in 225Ac-ch806–treated tumors compared with controls. Reduced Ki-67 expression was evident in all 225Ac-ch806–treated tumors. Increased expression of p21 and cleaved caspase 3 was shown in U87MG.de2-7 and DiFi tumors treated with 225Ac-ch806. Conclusion: In glioblastoma and colorectal tumor models, 225Ac-ch806 significantly inhibited tumor growth via induction of double-strand breaks, thereby constraining cancer cell proliferation while inducing cell cycle arrest and apoptosis. These findings underscore the potential clinical applicability of 225Ac-ch806 as a potential therapy for EGFR-expressing solid tumors.
Cell surface proteins constitute about 23% of the human proteome and play a crucial role in drug development (1). Around 66% of drugs approved for human use specifically target cell surface proteins (2). Despite the significant advancements in anticancer therapies achieved through targeted immunotherapy, challenges remain because some patients are unresponsive or develop resistance over time. To enhance efficacy and overcome resistance, 2 promising strategies have been developed: antibody–drug conjugates and radioimmunotherapy (3,4). Antibody–drug conjugates involve the conjugation of cytotoxic drugs to antibodies, whereas radioimmunotherapy uses antibodies labeled with radionuclides. Monoclonal antibodies, known for their high specificity and affinity to targets, enable antibody–drug conjugates and radioimmunotherapy to selectively deliver cytotoxic agents to disease sites, thereby minimizing side effects on normal tissues. So far, the U.S. Food and Drug Administration has approved 14 different antibody–drug conjugates and 2 radioimmunotherapy agents, including 133I-tositumomab and 90Y-ibritumomab, for the treatment of various cancers (3,5).
Radioimmunotherapy has shown promise in many studies; however, β-particle–emitting radionuclides can lead to bone marrow toxicity due to a combination of antibody circulation half-life, linear energy transfer, and penetrative distance of β-particles in tissue (6). Clinical studies have demonstrated the potential of α-particle–emitting 213Bi (half-life, 45.6 min) and 225Ac (half-life, 10 d) to treat hematologic malignancies, resulting in durable responses and minimal side effects (7). α-particle therapies using tumor-targeting peptides or small molecules also have displayed promising efficacy in both preclinical and clinical studies (8,9). α-particles cause more catastrophic DNA damage than β-particles (double-strand breaks [DSBs] vs. single-strand breaks) and can have less impact on surrounding healthy tissues because of their shorter penetrative distance (50–80 μm vs. 800–10,000 μm) (10,11).
Radioimmunotherapy agents require a specific monoclonal antibody, a suitable linker, and a radionuclide to restrain cancer cell growth. The ideal antibody should have high tumor uptake and low binding to normal tissues. Epidermal growth factor receptor (EGFR) is an attractive target because of its overexpression in many solid tumors; however, on-target side effects of EGFR-targeted therapies are common because of normal-tissue expression (12). To address this, a tumor-specific anti-EGFR monoclonal antibody, mAb806, showing specific targeting of EGFR-positive tumors in preclinical models and clinical studies has been developed and characterized (13–18). MAb806 bound to EGFR or EGFR.de2-7 mutant overexpressed in tumors but not to EGFR expressed in normal tissues, making it a promising antibody for radioimmunotherapy. In this work, ch806 (chimeric mAb806) is labeled with the α-emitting 225Ac to investigate the benefit of its DNA-based cell-killing mechanism. Optimal radiolabeling conditions to prepare 225Ac-ch806 are identified, and the biodistribution profile and therapeutic efficacy of 225Ac-ch806 are evaluated in preclinical models for glioblastoma and colorectal cancer.
MATERIALS AND METHODS
Reagents and Cell Lines
U87MG.de2-7 cells (with overexpression of EGFR.de2-7 mutant) were cultured in RPMI medium supplemented with 10% fetal bovine serum and antibiotics. DiFi cells (moderate expression of wild-type EGFR) were cultured in Dulbecco modified Eagle medium containing 10% fetal bovine serum and antibiotics.
H2macropa Conjugation, 225Ac Chelation, Radiochemical Yield, and Purity
Ch806 was buffer-exchanged into borate buffer (0.2 M, pH 9.0) via spin filtration. Bifunctional metal-ion chelator H2macropa-tzPEG3SqOEt dissolved in dimethyl sulfoxide was added to buffer-exchanged ch806 (final dimethyl sulfoxide concentration < 4%) and shaken overnight at 4°C and then at ambient temperature for 24 h. Excess reagents were removed, and conjugates were buffer-exchanged against sodium acetate (0.1 M, pH 5.5) via spin filtration. Conjugation of IgG1 isotype control antibody was performed using the same conditions. 225Ac stock solution (555 kBq, 30 μL) was added to solutions of H2macropa-tzPEG3Sq–conjugated monoclonal antibodies to give mixtures in a total volume of 100 μL with immunoconjugate concentrations of 10−6 M. Reaction mixtures were incubated at ambient temperature for 15 min, and samples were taken for determination of radiochemical purity and immunoreactive fraction. Apparent specific activities were calculated by dividing the starting activity of 225Ac by the amount of immunoconjugate and multiplying by radiochemical purity.
Additional methods on quality control of conjugated antibodies and radioimmunoconjugates can be found in the supplemental materials (available at http://jnm.snmjournals.org).
In Vitro Stability, Immunoreactivity, and Cytotoxicity
Serum stability was assessed by incubating radioimmunoconjugates (10 μg) in human serum from healthy donors (100 μL) at 37°C for 14 d. Radiochemical purity was measured by instant thin-layer chromatography. Immunoreactive fraction was determined by incubating radioimmunoconjugates with U87MG.de2-7 cells, followed by measurement of cell-bound activity via a γ-counter. To assay cytotoxicity, U87MG.de2-7 cells were treated with different constructs after serial dilution to indicated concentrations. Cells were incubated for 4 d (19), followed by measurement of cell viability by MTS reagent (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium).
Detailed methods on in vitro stability, immunoreactivity, and the MTS cytotoxicity assay can be found in the supplemental materials.
Biodistribution in Tumor-Bearing Mice
All animal studies were approved by the Austin Health Animal Ethics Committee. Four- to 6-wk-old female BALB/c nude mice were inoculated subcutaneously with 2.2 × 106 U87MG.de2-7 cells or 6 × 106 DiFi cells to establish corresponding xenograft models. Mice with established tumors (average tumor volume of ∼150 mm3 and ∼100 mm3 for U87MG.de2-7 and DiFi models, respectively) received intravenous injections of [225Ac]Ac-macropa-tzPEG3Sq-ch806 or [225Ac]Ac-macropa-tzPEG3Sq-IgG1 control. At designated time points after injection of radioimmunoconjugate, mice were humanely killed and biodistribution was assessed. Tumors and organs were collected and counted at secular equilibrium (minimum of 8 h after collection) in a γ-counter. Tissue distribution data were calculated as percentage injected dose per gram of tissue (%ID/g).
In Vivo Therapy Study
At 5 or 6 d after inoculation, mice bearing tumor xenografts (∼50–100 mm3) were injected intravenously with [225Ac]Ac-macropa-tzPEG3Sq-ch806, [225Ac]Ac-macropa-tzPEG3Sq-IgG1, or unlabeled ch806. Tumor volume was measured biweekly. Mice were humanely killed at an ethical endpoint (tumor volume > 1,000 mm3 or chronic body weight loss > 10%).
Additional methods on immunohistochemistry assays and dosimetry analysis can be found in the supplemental materials.
Statistical Analysis
Data were graphed and analyzed by Prism 8 (GraphPad). For multiple comparisons, 1-way ANOVA with Tukey multiple comparison testing was used.
General experimental information can be found in the supplemental materials.
RESULTS
225Ac-ch806 Synthesis, Radiochemical Yield and Purity, Apparent Specific Activity, Immunoreactivity, and Serum Stability
Optimal conditions for conjugation of chelators to the antibody and subsequent radiolabeling with 225Ac were investigated (Supplemental Figs. 1–6; Supplemental Table 1). Two different chelators including H2macropa-tzPEG3SqOEt and DOTA-methyltetrazine were investigated (Table 1). The radiochemical yield of [225Ac]Ac-macropa-tzPEG3Sq-ch806 surpassed that of [225Ac]Ac-DOTA-dhPzPEG4-ch806 at an equivalent chelator-to-antibody ratio (99.8% vs. 38.1% for a ratio of ∼2). Apparent specific activities were 36.9 MBq/mg and 1.41 MBq/mg for [225Ac]Ac-macropa-tzPEG3Sq-ch806 and [225Ac]Ac-DOTA-dhPzPEG4-ch806, respectively. The radiochemical purity and immunoreactivity of both constructs were comparable after synthesis. [225Ac]Ac-macropa-tzPEG3Sq-ch806 exhibited better stability in serum than [225Ac]Ac-DOTA-dhPzPEG4-ch806. The radiochemical purity of [225Ac]Ac-macropa-tzPEG3Sq-ch806 and [225Ac]Ac-DOTA-dhPzPEG4-ch806 was more than 97% and less than 75%, respectively, after 14 d in serum. The protein integrity of labeled and unlabeled immunoconjugates was confirmed, and the radiochemical stability of [225Ac]Ac-macropa-tzPEG3Sq-ch806 under La3+ and ethylenediaminetetraacetic acid challenge was excellent (Supplemental Figs. 7–8; Supplemental Tables 2–4). We therefore used [225Ac]Ac-macropa-tzPEG3Sq-ch806 (225Ac-ch806) and [225Ac]Ac-macropa-tzPEG3Sq-IgG1 isotype control (225Ac-IgG1) for all following studies.
Quality Control and Comparison of [225Ac]Ac-Macropa-tzPEG3Sq-ch806 and [225Ac]Ac-DOTA-dhPzPEG4-ch806
In Vitro Cytotoxicity Mediated by 225Ac-ch806
To investigate in vitro cytotoxicity of 225Ac-ch806, proliferation assays were performed with U87MG.de2-7 cells (Supplemental Fig. 9). Cells were incubated with ch806, free 225Ac, 225Ac-ch806, and 225Ac-IgG1 for 4 d. Incubation with ch806 did not affect cell viability, evidenced by approximately 100% viability at different concentrations. Free 225Ac and 225Ac-IgG1 demonstrated a moderate and comparable antiproliferative effect, suggesting that the cell-killing effect of 225Ac-IgG1 was nonspecific. In contrast, 225Ac-ch806 yielded a significant and dose-dependent antiproliferative effect, with reduced cell viability compared with the controls (Supplemental Fig. 9).
Biodistribution with Subcutaneous U87MG.de2-7 and DiFi Tumors
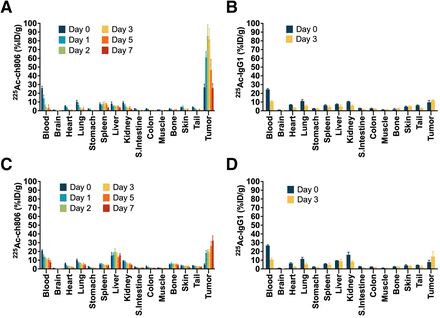
The ex vivo biodistribution of 225Ac-ch806 and 225Ac-IgG1 control was evaluated in U87MG.de2-7 (glioblastoma) and DiFi (colorectal) xenograft models. The biodistribution of 225Ac-ch806 was evaluated over time, from 0 (2 h) to 7 d after injection. In the U87MG.de2-7 model, tumor uptake of 225Ac-ch806 peaked on day 2 after injection, at 85.4 ± 12.7 %ID/g (Fig. 1A). General organ uptake was low, following the blood clearance pattern with some low-level accumulation in spleen and liver. Tumor uptake of 225Ac-IgG1 was 12.1 ± 0.5 %ID/g on day 3 after injection (Fig. 1B). As expected, clearance of 225Ac-IgG1 from blood and general organs was slower than that of 225Ac-ch806 because of lack of a target receptor. Excellent tumor-to-tissue ratios of 225Ac-ch806 in blood, liver, and muscle on day 3 after injection highlighted the exquisite target specificity of ch806 (Supplemental Fig. 10). These ratios were significantly higher than for 225Ac-IgG1, with a tumor-to-blood ratio of 1.1 ± 0.1, indicating no specific uptake in U87MG.de2-7 xenografts. These observations on tumor uptake and blood clearance were consistent with our previous study of 111In-ch806 biodistribution (20). Encouraged by these data, we evaluated the therapeutic efficacy of 225Ac-ch806 in this model.
Biodistribution of 225Ac-ch806 and 225Ac-IgG1 after injection in U87MG.de2-7 (A and B) and DiFi (C and D) xenografts. Data are expressed as mean ± SD (n = 5).
The biodistribution of 225Ac-ch806 in mice bearing DiFi tumors showed a steadily increasing tumor accumulation until day 7 after injection, with a maximum tumor uptake of 32.2 ± 5.5 %ID/g (Figs. 1C and 1D). DiFi cells displayed moderate EGFR expression compared with U87MG.de2-7 cells, as demonstrated by fluorescence-activated cell sorting (Supplemental Fig. 11). Although EGFR was not highly expressed in this cell line, the gradual increase in tumor uptake was consistent with constant turnover of EGFR.
Therapeutic Evaluation in U87MG.de2-7 and DiFi Xenograft Models
The therapeutic efficacy of 225Ac-ch806 was evaluated in mice bearing subcutaneous U87MG.de2-7 or DiFi tumors. The mice were treated with a single dose of 225Ac-ch806 (18.5 kBq, 0.5 μg), with 225Ac-IgG1 and ch806 used as controls. Survival, tumor burden, and body weight were monitored for 80 and 129 d, respectively.
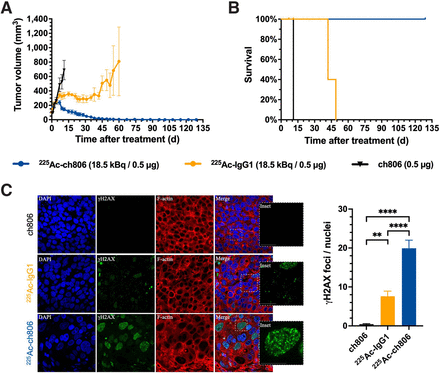
Tumor growth in the U87MG.de2-7 model was effectively inhibited by a single dose of 225Ac-ch806, with a long-lasting tumor-suppressive effect observed (Fig. 2A). Mice that received 225Ac-ch806 achieved 100% survival at the endpoint of this study (Fig. 2B). Median survival in the ch806- and 225Ac-IgG1–treated cohorts was 9 and 29 d after treatment, respectively. Both 225Ac-labeled antibodies caused a transient body weight loss, compared with the nonradioactive ch806 control (Supplemental Fig. 12A). Body weight loss was reversible and recovered to pretreatment levels. Tumor sections were harvested on day 6 after treatment, and analysis by immunofluorescence staining of γH2AX (molecular marker for DSBs) confirmed the presence of DSBs in mice treated with 225Ac-ch806. As anticipated, U87MG.de2-7 tumors from mice treated with 225Ac-ch806 exhibited a significantly higher level of DSBs than did tumors from the 225Ac-IgG1 and ch806 control groups (Fig. 2C).
Therapeutic study of 225Ac-ch806 in U87MG.de2-7 model. (A) Tumor growth curves of mice treated with 225Ac-ch806 (18.5 kBq/0.5 μg, n = 11), 225Ac-IgG1 (18.5 kBq/0.5 μg, n = 9), or ch806 (0.5 μg, n = 7). (B) Kaplan–Meier survival curves of mice bearing U87MG.de2-7 tumors. (C) Confocal microscopy of U87MG.de2-7 tumors stained with 4′,6-diamidino-2-phenylindole (blue) and antibodies against γH2AX (green) and F-actin (red). Average foci per nucleus from each treatment group were counted. Number of nuclei quantitated was >55. Scale bar = 20 μm. Data are expressed as mean ± SEM. *P ≤ 0.05.****P ≤ 0.0001. DAPI = 4′,6-diamidino-2-phenylindole.
Similar outcomes were observed in the DiFi model, despite relatively modest tumor accumulation of 225Ac-ch806 compared with U87MG.de2-7 xenografts. Tumor volumes in the 225Ac-ch806–treated cohort declined steadily from day 4 after treatment (Fig. 3A). At the study endpoint, 7 mice demonstrated a complete response and 1 mouse exhibited a partial response to treatment with 225Ac-ch806. Median survival in the ch806 and 225Ac-IgG1 control groups was 11 and 42 d after treatment, respectively (Fig. 3B). Both cohorts treated with 225Ac-labeled antibodies experienced a transient body weight loss that was reversible, eventually recovering to pretreatment levels (Supplemental Fig. 12B). The responses to 225Ac-ch806 were durable in both the U87MG.de2-7 and the DiFi models, an observation that was supported by the slow efflux rate of 225Ac-ch806 from tumor cells (Supplemental Fig. 13). Dosimetry analysis showed high doses delivered to U87MG.de2-7 and DiFi tumors and low doses delivered to normal tissues (Supplemental Fig. 14). Significantly higher DSBs were observed in DiFi tumors treated with 225Ac-ch806 than in the ch806 and 225Ac-IgG1 controls (Fig. 3C), providing further evidence of the impactful cellular response induced by 225Ac-ch806 treatment.
Therapeutic study of 225Ac-ch806 in DiFi model. (A) Tumor growth curves of mice treated with 225Ac-ch806 (18.5 kBq/0.5 μg, n = 14), 225Ac-IgG1 (18.5 kBq/0.5 μg, n = 9), or ch806 (0.5 μg, n = 7). (B) Kaplan–Meier survival curves of mice bearing DiFi tumors. (C) Confocal microscopy of DiFi tumors stained with 4′,6-diamidino-2-phenylindole (blue) and antibodies against γH2AX (green) and F-actin (red). Average foci per nucleus from each treatment group were counted. Number of nuclei quantitated was >60. Scale bar = 20 μm. Data are expressed as mean ± SEM. **P ≤ 0.01. ****P ≤ 0.0001. DAPI = 4′,6-diamidino-2-phenylindole.
Proliferation, Cell Cycle, and Apoptosis Analysis of U87MG.de2-7 and DiFi Tumors
To further study the mechanism of action of 225Ac-ch806 on inhibiting tumor growth, U87MG.de2-7 and DiFi tumors were subjected to hematoxylin and eosin, Ki-67, p21, and cleaved caspase 3 staining to assess tumor architecture, proliferation, cell cycle, and apoptosis. Tumors treated with 225Ac-ch806 exhibited reduced proliferation in both models, indicated by lower Ki-67 staining than in tumors treated with ch806 or 225Ac-IgG1 (Fig. 4). Given that U87MG.de2-7 tumor growth was hindered but not eradicated by 225Ac-ch806, we proceeded to examine cell cycle arrest in these tumors. We focused on expression of p21, a molecule that negatively regulates the cell cycle. Expression of p21 was notably stimulated by 225Ac-ch806, in contrast to the ch806 and 225Ac-IgG1 controls. These observations are consistent with the possibility that treatment with 225Ac-ch806 in U87MG.de2-7 led to cell cycle arrest through formation of DSBs. Because of the p53 status of DiFi cells (p53mut), p21 staining was not undertaken for DiFi tumors (21,22). Because most of the DiFi tumors were eradicated at the endpoint after treatment with 225Ac-ch806, we examined apoptosis in these tumors. DiFi tumors treated with 225Ac-ch806 exhibited notably elevated levels of apoptosis in comparison to those treated with ch806 and 225Ac-IgG1. This observation implies that 225Ac-ch806 can prompt cell death in DiFi tumors through apoptotic pathways.
Immunohistochemistry to study tumor architecture, proliferative capacity, and cell cycle at endpoint. (A and B) U87MG.de2-7 (A) and DiFi (B) tumors were stained for hematoxylin and eosin, Ki-67, and p21 or cleaved caspase 3. Scale bar = 2 mm in whole-tumor hematoxylin and eosin images and 200 μm in insets. (C) Quantitative analysis of Ki-67 and p21 or cleaved caspase 3 staining in both tumor models. *P ≤ 0.05. **P ≤ 0.01. ***P ≤ 0.001. ****P ≤ 0.0001. cCasp3 = cleaved caspase 3; H&E = hematoxylin and eosin.
DISCUSSION
The ch806 anti-EGFRvIII antibody was modified by attaching an H2macropa chelator using a bifunctional derivative bearing a squaramide ester moiety. Also investigated was an alternative 2-step bioconjugation strategy involving the use of a bioorthogonal reaction between DOTA-methyltetrazine and antibody modified to incorporate a trans-cyclooctene-PEG4 functional group. Efficient complexation of 225Ac by DOTA-methyltetrazine at elevated temperatures was followed by an inverse-electron-demand Diels–Alder reaction between [225Ac]Ac-DOTA-methyltetrazine and trans-cyclooctene-PEG4–modified antibodies at ambient temperature to conserve protein integrity (23).
Our results indicate the potential application of tumor-specific anti-EGFR ch806 antibody in targeted α-therapy for solid tumors. The wide expression of EGFR in various human tumor types, spanning the lung, colon, pancreas, breast, head and neck, bladder, kidney, and nervous system (24,25), positions it as an attractive target for cancer treatment. Although targeted therapies against EGFR have gained prominence as standard care for cancer patients with EGFR overexpression in tumors, challenges have persisted due to EGFR’s expression in normal tissue, including the skin and gut (26), leading to undesirable on-target side effects of EGFR-targeted therapies. MAb806 addresses this limitation and exclusively targets abnormally overexpressed EGFR and EGFRvIII mutant forms in tumors, excluding normal tissues (13–16,27,28). The tumor-targeting effect of mAb806 has been demonstrated in both preclinical and clinical studies (13,14,29–32). Another hurdle in EGFR-targeted therapy is tumor relapse, a phenomenon observed in many patients after treatment (33). The treatment options are limited after relapse from targeted therapies, and our results suggest that 225Ac-ch806–based targeted α-therapy may be able to address this issue.
This work demonstrates that 225Ac-ch806 exhibited a potent in vitro cell-killing effect in U87MG.de2-7 cells, affirming its ability to specifically target EGFR-expressing cancer cells and effectively induce cell death. Although modest cytotoxicity was observed for 225Ac-IgG1 and free 225Ac, this finding could potentially be attributed to the extended incubation time (4 d), leading to nonspecific cytotoxicity from radioactive culture medium. A previous study has reported that an extended incubation time can result in increased cytotoxicity of free 225Ac (34).
The high uptake of 225Ac-ch806 in subcutaneous glioblastoma and colorectal tumors, in contrast to 225Ac-IgG1 control, underscored the specificity of tumor targeting by this targeted α-therapy. The rapid blood clearance and increasing tumor uptake over time emphasized the tumor-targeting specificity of 225Ac-ch806. Furthermore, the uptake of radioactivity in liver was low, suggesting the stability of 225Ac-ch806, as free 225Ac accumulates predominantly in liver (35). The lack of normal-tissue targeting demonstrated in human trials of ch806 and humanized-form ABT-806 confirms the tumor-specific targeting of this antibody and potential for delivery of payloads to tumor (13,29). The profound in vivo antitumor activity of 225Ac-ch806 highlights its potential as a possible therapy for EGFR-expressing tumors. Notably, a single dose of 225Ac-ch806 induced a substantial therapeutic effect in both U87MG.de2-7 and DiFi xenograft models. Treatment responses were durable in both models, with prolonged survival to the study endpoint, which was likely supported by the slow efflux rate of 225Ac-ch806 (Supplemental Fig. 13). Given that relapse is often encountered in various targeted therapies, this outcome is particularly encouraging. In relation to recent data on anti-EGFR 225Ac-nimotuzumab, our study exhibited equivalent efficacy with the advantage of tumor-selective targeting for potential human trials (36). Dosimetry analysis showed high doses delivered to U87MG.de2-7 and DiFi tumors despite their small volume (Supplemental Fig. 14). This finding suggests potential efficacy of 225Ac-ch806 therapy in clinical small-volume disease such as metastatic cancers.
DSBs present in tumors treated with 225Ac-ch806 were identified by γH2AX staining. The cellular response to DNA damage involves a complex series of molecular events that can lead to either apoptosis or cell cycle arrest and subsequent senescence (37–39). The primary target of ionizing radiation, specifically for α-particle radiation, is DNA (40). 225Ac is exquisitely effective in inducing DNA damage due to high-linear-energy transfer and produces denser ionization events at the site of radioactive decay. DNA damage induced by α-particle emitters can be visualized by recruitment of γH2AX, resulting in distinct foci at DSB sites.
The observed radiation effects in tumors, characterized by distinctive features such as enlarged nuclei, atypical nuclei, and the emergence of intracellular fibrosis, have been corroborated by findings from other studies (41). It has been demonstrated that radiation can precipitate failures in the mitotic process, consequently leading to outcomes such as cell death or senescence (42). Specifically, after DNA damage, cells may fail in their attempts to complete the mitotic cycle, leading to the presence of atypical nuclei. Histologic assessments have unveiled the presence of substantial vacuoles within U87MG.de2-7 tumors subjected to 225Ac-ch806 treatment. The appearance of these vacuoles signifies a phenomenon known as methuosis, a form of cell death that deviates from the classic apoptotic pathway (43). This unique mechanism of cell death is characterized by the formation of extensive vacuoles within the cytoplasm.
CONCLUSION
We have shown the promising potential of 225Ac-ch806 for the treatment of EGFR-positive tumors. These results provide a solid foundation for potentially advancing 225Ac-ch806 toward clinical translation.
DISCLOSURE
This study was supported by the Australian government National Health and Medical Research Council (grant 1143710). Andrew Scott is supported by a National Health and Medical Research Council investigator grant (grant 1177837) and is an inventor on patents for mAb806. Andrew Scott, Christian Wichmann, Katherine Morgan, and Paul Donnelly are inventors on a patent for H2macropa-SqOEt. No other potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: Is 225Ac-ch806 therapy effective in EGFR-positive solid tumors?
PERTINENT FINDINGS: In a single-dose treatment, 225Ac-ch806 induced prolonged tumor growth inhibition in murine EGFR-overexpressing and wild-type EGFR xenograft models.
IMPLICATIONS FOR PATIENT CARE: Targeted α-therapy with 225Ac-ch806 is a promising strategy to treat EGFR-positive solid tumors.
ACKNOWLEDGMENTS
We thank the U.S. Department of Energy Isotope Program (managed by the Office of Isotope R&D and Production) for supplying 225Ac for this study, and we thank the Australian Cancer Research Foundation for funding to support nuclear magnetic resonance spectroscopy and the Bio21 Mass Spectrometry and Proteomics Facility of the University of Melbourne. The graphical abstract was prepared using BioRender and PDB entry codes 1IVO, 3G5X, 3G5V, and 3G5Y.
Footnotes
Published online Jul. 25, 2024.
- © 2024 by the Society of Nuclear Medicine and Molecular Imaging.
REFERENCES
- Received for publication October 29, 2023.
- Accepted for publication June 5, 2024.