Visual Abstract
Abstract
177Lu-DOTATATE is an effective second-line treatment for metastatic or nonresectable neuroendocrine tumors. This treatment can result in hematologic severe adverse reactions (SARs). Preemptive identification of patients at risk of SARs could mitigate this risk and improve treatment safety and outcomes. Methods: Demographic and oncologic history, pretreatment laboratory values, and SAR frequency were obtained for 126 sequential patients treated with 177Lu-DOTATATE. Univariable and multivariable logistic regression models identified factors correlating with SARs. Results: Relative pretreatment anemia, leukopenia, thrombocytopenia, and elevated mean corpuscular volume (MCV) were significantly correlated with SARs, with an odds ratio of 16 (95% CI, 5–65) in patients with an MCV greater than 95 fL. Conclusion: Pretreatment bone marrow dyscrasias, including an MCV greater than 95 fL, may predict patients at risk for SARs when treated with 177Lu-DOTATATE. Further study is needed to determine whether the risks of SARs outweigh the benefit in these patients.
Neuroendocrine tumors are an uncommon malignancy typically arising from the small bowel or pancreas. Metastatic disease is common at presentation, with few systemic treatment options for disseminated disease (1). The success of the targeted radiopharmaceutical therapy 177Lu-DOTATATE in the NETTER-1 trial has led to widespread clinical use (2).
Although effective, 177Lu-DOTATATE therapy can have severe and treatment-limiting adverse effects, with severe gastrointestinal and hematologic toxicities being reported in a minority of patients (3,4). Severe hematologic toxicities, although rare, are morbid, requiring transfusions or imparting significant susceptibility to bleeding or infection (5,6). Preemptively identifying patients at high risk of a hematologic severe adverse reaction (SAR) could help mitigate this risk. This retrospective analysis aimed to identify baseline parameters predictive of SARs before 177Lu-DOTATATE radiopharmaceutical therapy.
MATERIALS AND METHODS
Patients
We identified 126 consecutive adults with metastatic or unresectable neuroendocrine tumors who completed treatment (4 doses or termination due to disease progression or SARs) with 177Lu-DOTATATE at Johns Hopkins Hospital between January 2018 and August 2023.
Patient data were extracted from the electronic medical record. Laboratory values were obtained before each 177Lu-DOTATATE cycle and within 3 mo after therapy or at the initiation of an additional line of therapy, whichever occurred first. Adverse events were graded using the Common Terminology Criteria for Adverse Events 5.0, with a SAR defined as grade 3 or worse anemia, thrombocytopenia, or leukopenia. SARs were categorized as transient or permanent by persistence at 12-mo follow up.
The Johns Hopkins institutional review board (IRB00328627) approved this retrospective study and waived the requirement to obtain informed consent.
Statistical Analysis
Data analysis and logistic regressions were performed in R (version 4.3.1; Foundation for Statistical Computing) with a threshold of 0.05 for statistical significance.
RESULTS
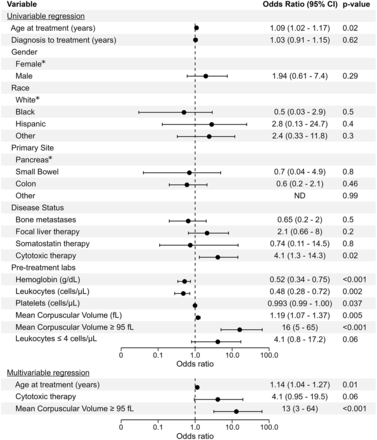
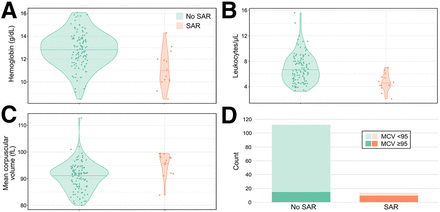
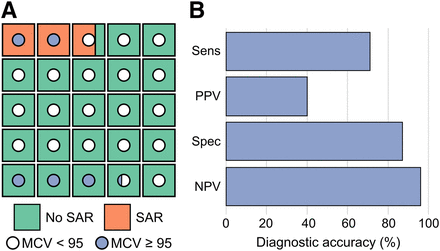
Of the 126 patients analyzed, 14 (11%) had SARs (Table 1). Baseline complete blood count, demographics, and treatment history were analyzed with a univariable logistic regression model (Fig. 1). Age, prior chemotherapy, and baseline hematologic parameters including hemoglobin, platelet count, leukocyte count, and mean corpuscular volume (MCV) correlated significantly with SARs (Table 1; Fig. 2). Notably, the presence of bone metastases, focal liver treatment, or somatostatin therapy did not correlate with SARs. Our univariable logistic regression analysis demonstrated that an MCV greater than 95 fL correlated more strongly with SARs than did other individual parameters. Age at initial treatment, prior cytotoxic therapy, and an MCV greater than 95 fL were included in a multivariable logistic regression (Fig. 1), and both age and an MCV greater than 95 fL correlated significantly with SARs. We found that an MCV threshold of 95 fL yielded a sensitivity of 71%, specificity of 87%, positive predictive value of 40%, and a negative predictive value of 96% (Fig. 3) for SARs.
Baseline Patient Characteristics
Forest plot of univariable (top) and multivariable (bottom) logistic regression evaluating factors correlated with development of SARs after 177Lu-DOTATATE. *Reference category.
Comparison of baseline hematologic parameters in patients with and without SARs: hemoglobin (A), leukocytes (B), MCV (C), and MCV ≥ 95 fL (D).
Diagnostic performance of MCV ≥ 95 fL in predicting SARs. (A) Prevalence of SARs and MCV ≥ 95 fL in patients treated with 177Lu-DOTATATE. Each box represents ∼5 patients. (B) Diagnostic performance statistics of MCV ≥ 95 fL in predicting SARs. NPV = negative predictive value; PPV = positive predictive value; sens = sensitivity; spec = specificity.
Overall, treatment was halted prematurely in 21% of patients because of progressive disease or death, which occurred in 16 (80%) of the non-SAR patients and 1 (14%) SAR patient. Six (86%) of the SAR patients halted treatment because of SARs, compared with 0% in the non-SAR group, whereas 3 (15%) of the non-SAR group had treatment-limiting non-SAR adverse events. Half the patients who developed SARs did so during treatment, although without a definite temporal pattern.
Patients with SARs fared poorly, with death or persistent myelosuppression at 1 y after treatment occurring in 7 (50%) and 4 (29%) patients, respectively. The risk of death or persistent myelosuppression is likely independent of the nature of both the initial hematologic dyscrasia and the pretreatment MCV, although the small number of patients with SARs may obscure subtle differences (Fig. 4).
One-year posttreatment outcomes in patients with SARs. (A) SAR outcomes based on initial hematologic toxicity. (B) Outcomes of patients with SARs by baseline MCV.
DISCUSSION
We performed a retrospective analysis on a cohort of patients with neuroendocrine tumors treated with 177Lu-DOTATATE and found that age, prior systemic chemotherapy, and multiple hematologic parameters, notably an MCV of 95 fL or higher, correlated with SARs. We, and others, identified a significant number of patients treated with 177Lu-DOTATATE who developed grade 3 or 4 lymphopenia (2). However, because lymphopenia after 177Lu-DOTATATE is almost always clinically inconsequential, lymphopenia was not included in this analysis.
In our cohort, pretreatment hematologic parameters correlated significantly with SARs. Although the correlation was strongest with an MCV greater than 95 fL, lower hemoglobin, leukocytes, and platelet values all correlated significantly with SARs. We hypothesize that many of the patients who ultimately develop SARs have preexisting, albeit subclinical, bone marrow dyscrasias that are exacerbated by treatment. Accordingly, age and history of chemotherapy also correlated with SARs—both of which are factors associated with myelotoxicity in other contexts (7,8). Curiously, the presence of bone marrow metastases was not associated with an increased risk of SARs. These results are in partial agreement with Bergsma et al., who found leukopenia and age to correlate with SARs. We also found a correlation between leukopenia and SARs; however, an elevated MCV yielded a higher odds ratio (4.1 vs. 16). Bergsma et al. did not identify an effect of prior chemotherapy, although this result may have been due to the paucity of such patients in their cohort (9).
Other 177Lu-DOTATATE cohorts experienced differing extents of SARs. Only 2% of patients in the NETTER-1 trial experienced grade 3 or 4 anemia or thrombopenia, compared with 11% in this study, 11% in a large Dutch cohort, and approximately 10% overall in a metaanalysis (9,10). The nature of this disparity is unclear; patients in this cohort met the hematologic inclusion criteria used in the NETTER-1 trial, and the age of patients was similar. Neither the pretreatment MCV nor the history of systemic chemotherapy was reported in the NETTER-1 trial. However, patients in this cohort likely had more advanced disease, indicated by slower progression to 177Lu-DOTATATE therapy than in the NETTER-1 patients (5.7 and 3.8 y, respectively) and a higher prevalence of bone metastases (52% and 11%), which may have predisposed patients to the development of SARs (10).
Our study had several limitations. First, the fact that it was a retrospective, single-center study may limit the generalizability of our results. Second, our population likely had more advanced disease than comparator cohorts. A third limitation is the relatively small number of patients who had SARs. However, we included all consecutive patients treated with 177Lu-DOTATATE, and our data may better reflect the patients seen in routine clinical practice. Further study is needed to determine whether combining additional measures would improve the accuracy of detecting patients at risk for SARs. Other radionuclide therapies have entered routine clinical use, namely 223Ra-dichloride and 177Lu-PSMA-617 for the treatment of prostate cancer. Both have been associated with myelosuppression and SARs in a minority of patients (11,12). The association of pretreatment macrocytosis has not been studied in these agents, and further study is needed to determine whether these findings are applicable beyond 177Lu-DOTATATE.
Pretreatment complete blood counts are routinely collected on patients undergoing treatment with 177Lu-DOTATATE; an MCV of 95 fL or higher is therefore a convenient screening parameter to identify patients at risk of SARs. Although bone marrow dyscrasias can lead to macrocytosis, the differential diagnosis for an elevated MCV is broad, including ethanol use, nutritional deficiencies, and medications, and not all etiologies may induce sensitivity to SARs (13). Accordingly, an MCV cutoff of 95 fL alone should not be used to exclude patients from 177Lu-DOTATATE therapy. Rather, we envision screening patients to identify those who might benefit from further pretreatment analysis. Ultimately, a composite factor of hematologic parameters or a tiered approach to assess the underlying marrow health in at-risk patients may prove more accurate and guide treatment decisions.
CONCLUSION
We evaluated factors associated with SARs in patients treated with 177Lu-DOTATATE and found a significant correlation with patient age, history of cytotoxic chemotherapy, and a pretreatment MCV of 95 fL or higher. An elevated MCV may herald 177Lu-DOTATATE–induced SARs; further study is needed to determine whether the risks of SARs outweigh the benefit in these patients.
DISCLOSURE
No potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: Do patient characteristics and baseline hematologic parameters predict SARs in neuroendocrine patients undergoing 177Lu-DOTATATE treatment?
PERTINENT FINDINGS: Retrospective analysis of 126 patients demonstrated that age, prior chemotherapy, and baseline hematologic parameters, including an MCV of 95 fL or higher, correlated significantly with 177Lu-DOTATATE–related SARs.
IMPLICATIONS FOR PATIENT CARE: Baseline hematologic parameters, including the MCV, correlate with an increased risk of SARs, and patients could benefit from additional pretreatment screening.
Footnotes
Published online Jul. 11, 2024.
- © 2024 by the Society of Nuclear Medicine and Molecular Imaging.
REFERENCES
- Received for publication January 17, 2024.
- Accepted for publication June 5, 2024.