Visual Abstract
Abstract
Myocardial somatostatin PET uptake is observed not only in most patients with acute myocarditis (AM) but also in some oncology patients referred for routine somatostatin PET. This raises concerns about the specificity of somatostatin PET for detecting myocarditis. The current study aims to identify factors associated with the detection of myocardial uptake on somatostatin PET scans recorded for oncology indications and differential PET criteria that characterize myocardial uptake in AM patients. Methods: We analyzed factors associated with the detection of myocardial [68Ga]Ga-DOTATOC uptake in 508 [68Ga]Ga-DOTATOC PET scans from 178 patients, performed for confirmed or suspected oncologic disease (Onc-PET) and PET criteria that could differentiate myocardial [68Ga]Ga-DOTATOC uptake in 31 patients with MRI-ascertained AM (AM-PET) from that in the Onc-PET group. Results: Significant myocardial uptake was detected in 137 (26.9%) Onc-PET scans and was independently associated with somatostatin analog treatment (exp(β), 0.805; 95% CI, 0.728–0.890; P < 0.001) and age (exp(β), 1.005; 95% CI, 1.001–1.009; P = 0.012). A comparable model was selected for predicting the myocardial-to-blood SUVmax ratio using somatostatin analog treatment (P < 0.001) and history of coronary artery disease (P = 0.022). Myocardial uptake was detected in 12.9% (25/193) of Onc-PET scans from patients treated with somatostatin analogs but in 43.4% (59/136) of untreated patients over the median age of 64 y. Myocardial uptake was apparent in all 31 AM-PET scans, with volume and intensity of uptake dramatically higher than in the 137 Onc-PET scans showing myocardial uptake. A myocardial-to-blood SUVmax ratio threshold of 2.20 provided a sensitivity of 87% (27/31) and a specificity of 88% (44/50) for differentiating myocardial uptake between the AM-PET group and an Onc-PET group restricted to patients with clinical characteristics comparable to those of patients in the AM-PET group (≤64 y of age, no coronary artery disease history, and no somatostatin agonists). A myocardial uptake volume threshold of 18 cm3 provided comparable diagnostic accuracy (sensitivity, 84% [26/31]; specificity, 94% [47/50]). Conclusion: Myocardial uptake was detected in 26.9% of somatostatin PET scans recorded for oncology indications. This rate was decreased by somatostatin analog treatments and increased in older individuals. However, somatostatin PET scans, analyzed with the quantitative criterion of uptake intensity or volume, are able to identify AM and to differentiate it from myocardial uptake of other origins.
Myocarditis is far from being infrequent. It sometimes mimics an acute coronary syndrome, with some cases presenting or evolving toward left ventricular (LV) dysfunction, heart failure, and arrhythmia (1).
An endomyocardial biopsy, analyzed using the Dallas criteria, remains the gold standard for myocarditis diagnosis, even though it has low sensitivity for detecting focal forms (2). MRI is an efficient noninvasive diagnostic tool when analyzed using the 2018 revised Lake Louise criteria (3). However, MRI suffers from low sensitivity for detecting subacute or chronic myocarditis, characterized by cardiomyopathylike or arrhythmia presentations (4).
[68Ga]DOTA peptides were developed for PET imaging of somatostatin receptor (SSTR) subtypes to diagnose or monitor neuroendocrine tumors. However, SSTRs are overexpressed in lymphocytes and activated macrophages, the primary cell subsets involved in myocarditis (1,2).
Previous pilot somatostatin PET studies have reported myocardial radiopeptide uptake in most patients with acute myocarditis (AM) (5–10). For reasons that are still poorly understood, myocardial uptake is also observed in numerous patients undergoing somatostatin PET for routine oncology indications (11–13), raising concerns about the specificity of somatostatin PET to identify AM.
This study aims to identify the factors associated with myocardial uptake observed on somatostatin PET scans recorded for routine oncology indications and define differential PET criteria that characterize myocardial uptake in AM patients.
MATERIALS AND METHODS
Study Populations
This study analyzed a retrospectively constituted group of patients with consecutive [68Ga]Ga-DOTATOC PET/CT scans recorded in our nuclear medicine department between January 2019 and December 2023 (Onc-PET group). Patients were referred from the Regional University Hospital Center of Nancy or the neighboring Regional Oncology Center (Institut de Cancerologie de Lorraine) to investigate conventional oncology indications. PET/CT scans from patients with carcinoid syndrome or a cardiac tumor location were excluded. We extracted the variables listed in Table 1 from electronic medical databases, including sex, age, tumor data, history of oncologic and cardiac interventions, ongoing oncologic treatments, and cardiovascular risk factors.
Main Data Recorded at Time of PET/CT in Overall Onc-PET Group, Onc-PET Subgroup with Myocardial Uptake, and AM-PET Group
We analyzed a second group of [68Ga]Ga-DOTATOC PET/CT patients recorded between July 2020 and April 2023 as part of a prospective study of AM ascertained by MRI according to the 2018 Lake Louise criteria (AM-PET group), with our CMR methodology being described in the supplemental materials (supplemental materials are available at http://jnm.snmjournals.org) (3,14). Both the AM-PET and the Onc-PET study protocols were approved by dedicated ethics committees (Comité de Protection des Personnes–Ouest IV and Comité d’Éthique du Centre Hospitalier Régional Universitaire de Nancy, respectively) and released on the ClinicalTrials.gov website under the identifiers NCT03347760 and NCT05478733, respectively. All participants from the AM prospective study signed an informed-consent form to participate. The requirement to obtain informed consent was waived for the Onc-PET patients, but they had been informed that their medical data could be used retrospectively for research purposes.
PET/CT Recording and Analysis
All PET scans were recorded 60 min after the intravenous injection of 2 MBq/kg of [68Ga]Ga-DOTATOC on a digital PET/CT system (Vereos; Philips). In patients treated with somatostatin analogs, the PET/CT procedures were scheduled before the monthly treatment dose (15).
Both Onc-PET and AM-PET scans were reconstructed with 2-mm3 voxels, a similar iterative algorithm, and additional corrections detailed elsewhere (5). A 3-min PET recording was scheduled per bed position for Onc-PET scans, and a single 15-min cardiac-centered bed position was scheduled for AM-PET scans.
Areas of LV myocardial uptake of [68Ga]Ga-DOTATOC were detected visually with an SUV scaling range of 0–3 (5) and localized according to 17-segment LV division (Supplemental Fig. 1) (16). These uptake areas were considered significant only when they involved at least 2 contiguous LV segments; were associated with both a myocardial-to-blood SUVmax ratio of more than 1.5, with this ratio determined using 2-cm-diameter spheric volumes of interest, and a right atrium center location for blood activity; and were located more than 1 cm from liver activity (5).
In addition, a myocardial PET uptake volume was quantified using isocontours and the same criteria as used for the visual analysis (i.e., myocardial-to-blood SUVmax ratio > 1.5 and >1-cm distance from liver activity). This volume was considered zero when no myocardial uptake was detected visually.
Statistical Analysis
Continuous variables are represented as medians, with interquartile ranges, and categoric variables are represented as frequencies and percentages. Two-group comparisons were performed with χ2 tests (or Fisher exact tests when more appropriate) for categoric variables and with Mann–Whitney tests. Receiver operating characteristic curves were additionally performed for continuous variables, and associations between continuous variables were tested with the nonparametric Spearman rank correlation method. The significance threshold was set at a P value of less than 0.05, and the closest-to-(0,1) corner approach was used to define the optimal threshold value from receiver operating characteristic curves.
In the Onc-PET group, generalized linear mixed-effects models using SAS software (SAS Institute) for repeat measurements were used to identify factors associated with significant myocardial [68Ga]Ga-DOTATOC uptake (dichotomous variable), among those listed in Table 1, and linear mixed-effects models for repeat measurements (mixed SAS procedure) were used to determine factors associated with the log-transformed myocardial-to-blood SUVmax ratio. This log transformation was required to meet the normality assumptions of the linear models. Odds ratios (generalized linear mixed-effects models) and exp(β) (mixed models) with 95% CIs were reported, and P values of 0.15 and 0.05 were used as thresholds for, respectively, entering and removing variables from the models. Log linearity and linearity assumptions for continuous variables in generalized linear mixed-effects and mixed models were checked using the restricted cubic splines method, with knot locations based on Harrell’s recommended percentiles (17).
All analyses were conducted using SAS version 9.4.
RESULTS
Analysis of the Onc-PET Group
In total, 511 consecutive [68Ga]Ga-DOTATOC PET/CT scans were considered. Three were excluded because of confirmed or suspected cardiac metastasis or a technical PET problem, leaving 508 [68Ga]Ga-DOTATOC PET recordings from 178 patients in the current analysis. Seventy-six patients had a single PET scan, 56 patients had 2–3 PET scans, and 46 patients had more than 3 PET scans.
As detailed in Table 1, 423 (83.1%) of the Onc-PET scans were recorded from patients with a confirmed neuroendocrine tumor, but only 40 (7.9%) scans were recorded from patients with a history of coronary artery disease (CAD; myocardial infarction or coronary revascularization). Oncology surgery was documented in 266 (52.3%) cases, and the Onc-PET scans were recorded during periods of analog somatostatin treatment in 193 (37.9%) cases.
Significant myocardial uptake was detected in 137 (26.9%) Onc-PET scans and, as detailed in Table 2, the multivariate predictors of this uptake were somatostatin analog treatment (exp(β), 0.805; 95% CI, 0.728–0.890; P < 0.001) and age (exp(β), 1.005; 95% CI, 1.001–1.009; P = 0.012). A comparable model was selected for predicting the logarithm of the myocardial-to-blood SUVmax ratio—that is, somatostatin analog treatment (P < 0.001) and CAD history (P = 0.022).
Univariate and Multivariate Predictors of Myocardial Uptake and Log-Transformed Myocardial-to-Blood SUVmax Ratio in Onc-PET Group
The detection rate of myocardial uptake was only 12.9% (25/193) in somatostatin analog–treated patients. This rate was higher, 29.4% (53/180), when the absence of somatostatin analog treatment was associated with an age of no more than 64 y, which was the median of the Onc-PET population, and was even higher, 43.4% (59/136), when associated with the older age group. These data are illustrated in the graphical abstract.
Analysis of the AM-PET Group
Thirty-one patients were recruited at the time of AM diagnosis, with elevated plasma troponin I levels (median peak, 12.0 ng/mL; interquartile range, 6.9–17.4 ng/mL) and chest pain in 27 cases. Symptoms evocative of a gastrointestinal or respiratory tract infection were documented for 15 of these patients, and a recent (≤3 d) history of messenger RNA–based coronavirus disease 2019 vaccination was documented for 3 other patients. Ten AM patients had an abnormal LV ejection fraction (<50%) on MRI. The PET scan was performed a median of 4 d (range, 3–5 d) after peak troponin.
As detailed in Figure 1, the PET parameters considered (myocardial-to-blood SUVmax ratio and myocardial uptake volume) were unrelated to the MRI parameters used for tissue characterization (T1, T2, and the number of segments with late gadolinium enhancement). However, the LV ejection fraction, determined by MRI, was inversely correlated to the myocardial-to-blood SUVmax ratio (P = 0.008) and myocardial PET uptake volume (P = 0.013).
(A) Spearman correlation coefficients for associations between tissue characterization parameters provided by PET (myocardial-to-blood SUVmax ratio and myocardial uptake volume) or MRI (T1, T2, and number of late gadolinium enhancement [LGE] LV segments) and all considered PET and MRI variables. (B) Relationship between LV ejection fraction and myocardial-to-blood SUVmax ratio. (C) Relationship between LV ejection fraction and myocardial uptake volume.
Comparison Between the Onc-PET and the AM-PET Groups
As shown in Table 1, men were overrepresented in the AM-PET group compared with the Onc-PET group with myocardial uptake. Patients in the AM-PET group were also younger and had lower rates of most cardiovascular risk factors. None of the AM-PET group patients had a history of cancer or cardiac disease.
Significant myocardial uptake was detected in all 31 AM-PET scans, with the volume and intensity of uptake dramatically higher than in the 137 Onc-PET scans with myocardial uptake. The respective medians in these 2 groups were 2.80 (range, 2.29–3.00) and 2.00 (range, 1.76–2.18; P < 0.001) for the myocardial-to-blood SUVmax ratio and 47.3 cm3 (range, 24.0–135.0 cm3) and 2.2 cm3 (range, 0.6–9.5 cm3; P < 0.001) for the myocardial uptake volume (Fig. 2). Higher rates of myocardial uptake were also observed in the AM-PET group than in the Onc-PET group in a per-segment analysis, with the difference particularly marked for inferior and inferior-lateral segments (Fig. 2).
(A–C) Comparison of myocardial uptake in AM-PET and Onc-PET groups in terms of myocardial uptake volume (A), myocardial-to-blood SUVmax ratio (B), and myocardial uptake detection rate on each of 17 LV segments (C). Difference in uptake detection rates between AM-PET and Onc-PET groups is particularly marked (P < 0.001) in inferior and inferior-lateral segments (columns delimited with dashed red lines) and weaker (P = ∼0.05) in anterior and anterior-septal segments located in basal and median parts of LV (columns delimited with dashed blue lines).
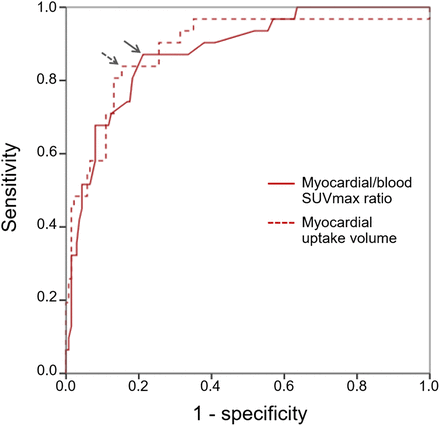
On the receiver operating characteristic curve analysis shown in Figure 3, both the myocardial uptake volume and the myocardial-to-blood SUVmax ratio were able to differentiate myocardial uptake in the AM-PET group from that in the Onc-PET group, with areas under the curve of 0.888 (95% CI, 0.817–0.960) and 0.879 (95% CI, 0.814–944), respectively. The criteria of a myocardial-to-blood SUVmax of more than 2.2 allowed differentiation of myocardial uptake between the AM-PET and the Onc-PET groups with a sensitivity of 87% (27/31) and a specificity of 77.4% (106/137). The criteria of a myocardial uptake volume of more than 18 cm3 provided comparable results (sensitivity, 84% [26/31]; specificity, 83% [114/137]).
Receiver operating characteristic curves optimized to differentiate AM-PET group from Onc-PET group on basis of myocardial-to-blood SUVmax ratio and myocardial uptake volume. According to closest-to-(0,1) corner approach, we selected thresholds of 2.2 for myocardial-to-blood SUVmax ratio and 18 cm3 for myocardial uptake volume (arrows).
Specificities of 88% (44/50) for the criteria of a myocardial-to-blood SUVmax ratio of more than 2.2 and of 94% (47/50) for that of a myocardial uptake volume of more than 18 cm3 were obtained when the Onc-PET group with myocardial uptake was restricted to a subgroup with characteristics more comparable to those of the AM group (i.e., age ≤ 64 y, no CAD history, and no somatostatin agonists).
Representative AM-PET and Onc-PET group scans are shown in Figure 4.
Representative fused PET/CT slices, on 0-to-2.5 SUV scale, of 41-y-old man in AM-PET group (diffuse and intense LV uptake) and of 3 Onc-PET patients with myocardial uptake: 71-y-old man with history of inferior myocardial infarction (MI; arrows mark uptake on inferior and inferoseptal segments),76-y-old woman with history of percutaneous transluminal angioplasty (PTCA; arrows mark uptake on proximal parts of anterior and septal walls), and 81-y-old man with no cardiovascular (CV) history (diffuse LV uptake). Myocardial-to-blood SUVmax ratio was 4.0 in AM-PET patient and ranged from 2.13 to 2.18 in Onc-PET patients.
DISCUSSION
Our study found that up to 26.9% somatostatin PET scans recorded for routine oncology indications show significant myocardial uptake. This is consistent with the prevalence reported in the literature (11–13). The uptake detection rate was decreased by somatostatin analogs and increased with patient age. Moreover, uptake in oncology cases was generally less intense and less extensive than uptake measured in AM cases, which supports the notion that somatostatin PET may be able to specifically detect AM.
Results obtained in our AM-PET group, where [68Ga]Ga-DOTATOC PET scans were recorded in MRI-ascertained AM cases, confirm our previously published preliminary results (5)—that is, consistently observed increased myocardial uptake relative to the blood activity on at least 2 contiguous LV segments, associated with a marked decrease in blood activity, and with myocardial uptake predominantly localizing to inferior and inferior-lateral LV segments (Fig. 2). The main technical limitations remain the relatively low activity of the myocardial uptake areas (mean SUVmax, 1.8 in the present AM-PET group) and the inability to analyze certain inferior LV segments proximal to liver activity.
In [68Ga]Ga-DOTATOC PET scans recorded for oncology indications, differences in SUVmax between organs from the same patient were previously shown to correlate with differences in SSTR expression (18). However, tissue-to-blood SUV ratios of [68Ga]Ga-DOTATOC are known to be a more accurate measure of SSTR density than SUV (19), and in the present study, the myocardial-to-blood SUVmax ratio was a better predictor of AM than was SUVmax. The lower blood SUVmax observed in the AM-PET group than in the Onc-PET group is also likely to enhance the difference in the myocardial-to-blood SUVmax ratio between the 2 groups.
The specificity of detecting AM could be undermined by the frequent observation of myocardial uptake in somatostatin PET recordings of common oncology indications. Such incidental observations were previously found to correlate with older patient age, a CAD history, and cardiovascular risk factors (11). In our large sample of [68Ga]Ga-DOTATOC PET scans recorded for an oncology indication, we also found a significant correlation between age and detection of myocardial uptake, as well as between a CAD history and the myocardial-to-blood SUVmax ratio. However, these 2 cardiac PET parameters were primarily associated with ongoing somatostatin antagonist treatment. Somatostatin analogs were prescribed in up to 37.9% of Onc-PET group cases, which is in line with their antiproliferative and antisecretory action in neuroendocrine tumors.
The myocardial uptake rate was only 12.9% in Onc-PET scans recorded in patients treated with somatostatin analogs. According to current recommendations (15), an Onc-PET scan was scheduled a few days before the monthly administration of somatostatin agonists and thus as long as 4 wk after the last administration. Nevertheless, such long-acting treatments are known to decrease tracer uptake in somatostatin PET for up to 25 d after their administration (20), and a primary consequence of this reduced uptake is an apparent increase in blood activity (as shown in the graphical abstract).
The detection rate of significant myocardial uptake was thus even higher when considering only the population not treated with somatostatin analogs and specifically the older subgroup of this cohort (43.4% uptake in the >64-y-old subgroup). This observation is not fully understood, although it is supported by the general concept that cardiovascular aging involves an overall increased level of heart inflammation (21).
Consistent with previous reports (6,22), we also observed myocardial uptake in patients with a history of myocardial infarction (Fig. 3). The previous observations of SSTR expression in fibroblasts could explain the detection of somatostatin PET uptake within myocardial scars (23,24). However, of the 24 Onc-PET scans from patients that presented a history of myocardial infarction, myocardial uptake was documented only in 10 cases. Certain areas of somatostatin PET uptake could also occur within the atherosclerotic plaques of coronary arteries, as previously reported (25). The anterior and anterior-septal segments, located in the basal and median parts of the LV, exhibited the slightest difference in uptake rate between the AM-PET and the Onc-PET groups (Fig. 2). However, this uptake location was previously considered to correspond to the atheromatous left anterior descending arteries (the PTCA history patient in Fig. 4). (26).
The main finding of the present study is that AM-PET is best differentiated from Onc-PET on the basis of the objective quantitative criteria of uptake intensity and volume—that is, myocardial-to-blood SUVmax ratio and myocardial uptake volume. These parameters were strongly interrelated in the present study population (Fig. 1), and using optimized thresholds, respective sensitivities of 87% and 84% were achieved. The respective specificities were 88% and 94% when the Onc-PET group with myocardial uptake was restricted to a subgroup with clinical characteristics more comparable to those of the AM group. Patients from this subgroup did not exhibit factors previously shown to affect myocardial uptake in our Onc-PET population, factors that are unlikely to be present in AM patients (>64 y old, somatostatin analog treatment, and history of CAD).
Our results appear to be more satisfactory than those previously published with [18F]FDG PET for detecting myocarditis (27). This most likely results from the difficulties of suppressing physiologic myocardial [18F]FDG uptake, despite diet or fasting. No preparation is recommended before a cardiac somatostatin PET scan except the withdrawal of corticosteroid or somatostatin analog treatment. Finally, the pathologic significance of the myocardial-to-blood SUVmax ratio and the myocardial uptake volume are strengthened by our additional observations of inverse associations with the LV ejection fraction in myocarditis patients (Fig. 1).
Our results need to be confirmed in other populations and require a dedicated cardiac somatostatin PET recording protocol for control groups. It would be interesting to determine whether this PET methodology could also detect cardiac sarcoidosis or chronic forms of myocarditis and to what extent our findings may be extrapolated to other somatostatin PET tracers, such as [68Ga]Ga-DOTANOC (28).
CONCLUSION
Significant myocardial uptake is observed in up to 26.9% of somatostatin PET scans recorded for routine oncology indications. This rate is even higher in the absence of somatostatin agonist treatments, particularly in older individuals. However, somatostatin PET scans, analyzed with the objective quantitative criterion of uptake intensity or volume, are able to identify AM and differentiate it from myocardial uptake of other origins.
DISCLOSURE
The sponsor was the Regional University Hospital Center (Centre Hospitalier Régional Universitaire) of Nancy, and this study was supported by a grant from the French Ministry of Health (APJ 2015) and by Advanced Accelerator Applications, a Novartis company, which provided the SomaKit TOC free of charge. No other potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: What are the parameters associated with the detection of myocardial uptake on somatostatin PET scans recorded for routine oncology indications, and what are the PET criteria for differentiating from AM?
PERTINENT FINDINGS: Myocardial uptake is frequently detected on oncologic somatostatin PET scans, especially in older patients not treated with somatostatin analogs, but this uptake is mostly less extensive and less intense than that observed in AM.
IMPLICATIONS FOR PATIENT CARE: Somatostatin PET scans, analyzed with the objective quantitative criterion of uptake intensity or volume, have the potential to diagnose AM and differentiate it from myocardial uptake of other origins.
ACKNOWLEDGMENTS
We thank Dr. Petra Neufing for critically reviewing the article and the staff of Nancyclotep for technical support.
Footnotes
Published online Jul. 11, 2024.
- © 2024 by the Society of Nuclear Medicine and Molecular Imaging.
REFERENCES
- Received for publication December 7, 2023.
- Accepted for publication May 22, 2024.