Visual Abstract
Abstract
Despite their unique histologic features, gliosarcomas belong to the group of glioblastomas and are treated according to the same standards. Fibroblast activation protein (FAP) is a component of a tumor-specific subpopulation of fibroblasts that plays a critical role in tumor growth and invasion. Some case studies suggest an elevated expression of FAP in glioblastoma and a particularly strong expression in gliosarcoma attributed to traits of predominant mesenchymal differentiation. However, the prognostic impact of FAP and its diagnostic and therapeutic potential remain unclear. Here, we investigate the clinical relevance of FAP expression in gliosarcoma and glioblastoma and how it correlates with 68Ga–FAP inhibitor (FAPI)–46 PET uptake. Methods: Patients diagnosed with gliosarcoma or glioblastoma without sarcomatous differentiation with an overall survival of less than 2.5 y were enrolled. Histologic examination included immunohistochemistry and semiquantitative scoring of FAP (0–3, with higher values indicating stronger expression). Additionally, 68Ga-FAPI-46 PET scans were performed in a subset of glioblastomas without sarcomatous differentiation patients. The clinical SUVs were correlated with FAP expression levels in surgically derived tumor tissue and relevant prognostic factors. Results: Of the 61 patients who were enrolled, 13 of them had gliosarcoma. Immunohistochemistry revealed significantly more FAP in gliosarcomas than in glioblastomas without sarcomatous differentiation of tumor tissue (P < 0.0001). In the latter, FAP expression was confined to the perivascular space, whereas neoplastic cells additionally expressed FAP in gliosarcoma. A significant correlation of immunohistochemical FAP with SUVmean and SUVpeak of 68Ga-FAPI-46 PET indicates that clinical tracer uptake represents FAP expression of the tumor. Although gliosarcomas express higher levels of FAP than do glioblastomas without sarcomatous differentiation, overall survival does not significantly differ between the groups. Conclusion: The analysis reveals a significant correlation between SUVmean and SUVpeak in 68Ga-FAPI-46 PET and immunohistochemical FAP expression. This study indicates that FAP expression is much more abundant in the gliosarcoma subgroup of glioblastomas. This could open not only a diagnostic but also a therapeutic gap, since FAP could be explored as a theranostic target to enhance survival in a distinct subgroup of high-risk brain tumor patients with poor survival prognosis.
Glioblastoma multiforme, a highly malignant primary brain tumor, has a median survival of about 14 mo after optimal surgical resection and chemoradiotherapy (1). Its subtype, gliosarcoma, shows both glial and sarcomatous differentiation (2,3) and has a similar median survival of approximately 17 mo (4). Despite their different histologies, both are treated with the same standard therapies (5). For simplicity, the term glioblastoma will be used for glioblastoma without sarcomatous differentiation hereafter, even though gliosarcomas are naturally considered a subgroup of glioblastoma.
Recent focus on the tumor microenvironment has been to find new therapies, despite the lack of survival benefits from previous immunotherapy trials in glioblastoma. Current studies explore potential immunotherapies (e.g., NOA-16, NOA-21, or DCVax trials (6–9)). Targeting molecules such as human epidermal growth factor receptor 2 or endothelial growth factor receptor in immunotherapy has been suggested, though their presence in normal tissues raises concerns about side effects and their varied expression in glioma cells hinders efficacy (10,11).
Fibroblast activation protein (FAP), found in a specific subgroup of tumor-associated fibroblasts known as cancer-associated fibroblasts, promotes tumor growth and invasion (12). FAP, an unusual serine protease with dipeptidyl peptidase and endopeptidase activities, influences the extracellular matrix and cellular signaling, altering gene transcription related to cell cycle, proliferation, and invasion (13,14). FAP also contributes to an immunosuppressive tumor microenvironment and can confer resistance to temozolomide (14). FAP’s regulation is influenced by factors such as transforming growth factor β-1 from astrocytes and glioma cells (15).
Although FAP is minimally expressed in normal tissues, its significant expression in glioblastoma, especially gliosarcoma, is likely linked to its mesenchymal differentiation (16–18). Ebert et al. observed that nearly 40% of glioblastomas were FAP-positive, whereas normal brain tissue was not (10). In vivo FAP-specific PET imaging shows higher FAP expression in more malignant tissues than in lower-grade gliomas, independent of cell counts (19–21). Inhibition of FAP leads to cell cycle arrest and decreased cell proliferation (22), yet the prognostic significance of FAP in glioblastoma and gliosarcoma remains uncertain.
MATERIALS AND METHODS
Study Design and Population
This retrospective analysis was conducted at the University Hospital Essen in Germany to investigate tumor tissue’s FAP expression in patients diagnosed with isocitrate dehydrogenase wild-type glioblastoma or gliosarcoma and poor overall survival prognosis, with a life expectancy not exceeding 2.5 y after primary diagnosis. Only patients who died within 2.5 y of diagnosis were included in this study, as there is a great therapeutic need because of the still high rate of negative phase III studies in glioblastoma, especially for patients who are not long-term survivors (23). The aim of the study was therefore to evaluate a potential tumor-specific target protein for radiopharmaceutical therapy for most glioblastoma and gliosarcoma patients who are not among the rare long-term survivors of more than 2.5 y. In one of the largest phase III studies on glioblastoma published to date to our best knowledge, only 1 in 5 patients reached this mark (24). The study was approved by the local ethics committee (approval number 19-9092-BO). We analyzed the correlation between immunohistochemical FAP expression and tracer uptake in 68Ga-FAP inhibitor (FAPI)–46 PET. Therefore, all glioblastoma patients who received a 68Ga-FAPI-46 PET scan at the University Hospital Essen and whose tissue was available for further analyses were included. The studies involving 68Ga-FAPI-46 PET imaging and the collection of patient-specific clinical data received approval from our institution’s ethics committee (approval numbers 19-8991-BO for the observational study and 20-9485-BO for the supplemental study). Moreover, all participants provided their informed consent by signing consent forms, ensuring adherence to ethical standards and respect for patient autonomy. The study was conducted in accordance with the Declaration of Helsinki and national regulations.
FAP Immunohistochemistry Evaluation
We performed immunohistochemical staining on a BenchMark ULTRA Slide Staining System (Roche) using anti-FAP α-antibody (Abcam, ab227703, clone SP325, monoclonal, rabbit) diluted 1:100. According to the data sheet, the antibody is reactive with human tissues with no reported cross reactivity. We validated this antibody in our laboratory using positive and negative controls, confirming the lack of cross reactivity and supporting specificity for intended targets (detailed data not included). Antigen retrieval occurred for 60 min at 36°C with cell conditioning-1 buffer (Roche). FAP expression was scored from 0 to 3 according to a semiquantitative FAP immunopositivity scoring system with slight modifications as previously described (Table 1; Fig. 1) (25,26).
Semiquantitative FAP Scoring System
Semiquantitative FAP immunopositivity scoring examples. (A) Score 0: tumor negative (glioblastoma). (B) Score 1: <10% of cells with perivascular staining (glioblastoma). (C) Score 2: ∼30% tumor cells positive (glioblastoma). (D) Score 3: >50% of tumor cells positive (gliosarcoma).
68Ga-FAPI-46 PET Imaging
We assessed imaging data in 15 patients as part of an observational trial (registered at clinicaltrials.gov under NCT04571086). We included patients from this study with suspected glioblastoma who underwent a 68Ga-FAPI-46 PET scan before surgery and subsequent definitive histopathologic diagnosis.
PET scans were performed in the craniocaudal direction on a Biograph mMR or Biograph Vision 600 scanner (Siemens Healthineers). The mean injected activity was 115.2 ± 41.5 MBq. 68Ga-FAPI-46 PET images were captured 20.2 ± 17.9 min after injection. PET images acquired with the Biograph mMR were reconstructed using an ordinary Poisson ordered-subset expectation maximization algorithm (3 iterations, 21 subsets) and a voxel size of 2.09 × 2.09 × 2.03 mm. A 4-mm gaussian filter was used for postsmoothing. Attenuation correction was performed using a Dixon-based segmentation approach. All PET/CT images were iteratively reconstructed using an ordinary Poisson ordered-subset expectation maximization algorithm (4 iterations, 5 subsets) using a time-of-flight option and a voxel size of 3.3 × 3.3 × 3.0 with a 4-mm postsmoothing gaussian filter. Images were reconstructed using dedicated manufacturer’s software (syngo MI.PET/CT; Siemens Healthineers). Low-dose CT was acquired for attenuation correction (30 mAs, 120 keV, 512 × 512 matrix, 3-mm slice thickness) in the case of CT imaging. SUVmax, SUVmean, and SUVpeak of intracranial lesions were measured with a region-growing algorithm with a threshold of 40% of the maximal uptake (Syngo.via software; Siemens Healthcare). PET/CT and PET/MR images were read by an experienced, masked nuclear medicine physician. For all patients, a preoperative MRI study was available. An automated postprocess coregistration was performed to match MRI with 68Ga-FAPI-46 PET scans (Syngo.via software; Siemens Healthcare).
For the exclusively intracranially located lesions, SUVmax, SUVmean, and SUVpeak were determined using a region-growing algorithm with a threshold set at 40% of maximum uptake. The study sought to determine the relationship between the semiquantitative FAP score in tissues, the PET data (SUV and tumor-to-brain ratio [TBR]), and the expression and uptake of FAP in tissues as revealed by the 68Ga-FAPI-46 PET scan.
Statistical Analysis
Progression-free and overall survival were analyzed using the Kaplan–Meier method, with a Spearman rank correlation to assess the relationships between clinical data and FAP expression or uptake. Patients still alive or who had survived beyond 2.5 y as of March 12, 2023, were excluded. Data analysis was performed with SPSS (IBM) and Prism (GraphPad) software. Progression-free survival was defined as the period from the initial tumor resection to the time of first recurrence, as identified by MRI. Overall survival spanned the time from the date of the first tumor resection and histopathologic diagnosis to the date of death. Patients who did not show progression in MRI follow-ups were censored and remained part of the study cohort.
RESULTS
Patient Characteristics
The FAP score was analyzed using tissue specimens from 13 gliosarcoma patients and 46 glioblastoma patients. In the glioblastoma group, 19 tumors (41%) exhibited O6-methylguanine-DNA methyltransferase (MGMT) promoter methylation, whereas in the gliosarcoma group, 2 tumors (15%) were MGMT promoter–methylated. For the glioblastoma cohort, the median initial Karnofsky performance status index stood at 70%, ranging from 40% to 100%. The gliosarcoma cohort had a median Karnofsky performance status index of 80%, ranging between 40% and 90%. Within the glioblastoma group, 17 patients (37%) experienced a complete surgical resection, characterized by the lack of any contrast-enhancing lesions in postoperative MRI scans taken within 72 h after the surgery. Conversely, 24 patients (52%) underwent a partial resection, and 3 patients (7%) had only a biopsy. For 2 patients, the extent of the resection could not be determined because of the unavailability of postoperative MRI scans. For the gliosarcoma group, complete resection was performed on 3 patients (23%) and partial resection on 9 patients (69%). One patient’s resection extent remained undetermined because of the absence of postoperative MRI scans. Furthermore, 15 eligible glioblastoma patients underwent a 68Ga-FAPI-46 PET scan using 68Ga-FAPI-46 before their surgical resection. Patient characteristics are provided in Table 2.
Patient Characteristics
FAP Expression in Tumor Tissue
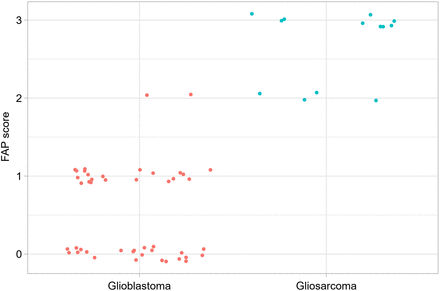
The expression of FAP was notably higher in gliosarcoma (median FAP score, 3) than in glioblastoma (median FAP score, 0.5) (P < 0.0001; Fig. 2). No glioblastoma samples achieved a maximum FAP score of 3, whereas gliosarcoma samples scored neither 0 nor 1. Glioblastoma with MGMT methylation had a median FAP score of 1 and without methylation had a median FAP score of 0. Gliosarcoma with MGMT methylation had a median FAP score of 2.5 and without methylation had a median FAP score of 3. In glioblastoma, FAP was expressed in perivascular regions, isolated tumor cells, and meninges. In gliosarcoma, abundant FAP was noted in mesenchymal tumor cells and perivascular regions. The study findings highlight differential FAP expression patterns between these brain tumors, with implications for diagnosis and treatment.
In gliosarcoma (green dots), FAP expression is more pronounced than in glioblastoma (red dots). Median FAP score in gliosarcoma was 3 (fractions: FAP score 2, n = 4 ≙ 31%; FAP score 3, n = 9 ≙ 69%) compared with 0.5 (fractions: FAP score 0, n = 23 ≙ 50%; FAP score 1, n = 21 ≙ 46%; FAP score 2, n = 2 ≙ 4%) in glioblastoma (P < 0.0001).
Survival Analyses
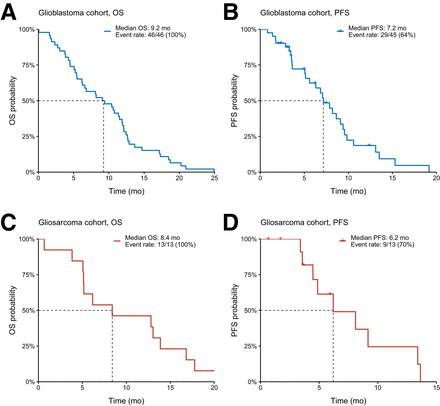
The glioblastoma cohort reported a median overall survival of 9.2 mo and a progression-free survival of 7.2 mo. The gliosarcoma cohort had slightly lower median overall and progression-free survival of 8.4 and 6.2 mo, respectively (Fig. 3). In a detailed breakdown, 46 glioblastoma patients were divided into 3 subgroups on the basis of FAP scores: 0 (n = 23), 1 (n = 21), and 2 (n = 2), with median overall survivals of 9.1, 8.2, and 15.9 mo, respectively (P = 0.54). Progression-free survival was 5.6, 9.6, and 10.2 mo for these groups (P = 0.07), indicating no significant correlation between the FAP score and survival. In the 13 gliosarcoma patients, those with a FAP score of 2 (n = 4) had a median overall survival of 4.4 mo, whereas those with a score of 3 (n = 9) lived longer, at 12.8 mo (P = 0.16). Progression-free survival was 8.5 mo for a FAP score of 2 and 6.2 mo for a FAP score of 3 (P = 0.95), also showing no significant correlation between FAP score and prognosis (Supplemental Fig. 1; supplemental materials are available at http://jnm.snmjournals.org). Additionally, glioblastoma patients with MGMT methylation had a median overall survival of 5.5 mo, surprisingly lower than the 11.4 mo for those without methylation, likely due to the study’s limitation of including only patients who lived no longer than 2.5 y after diagnosis. In gliosarcomas, the median overall survival was 10.9 mo for MGMT-methylated patients compared with 8.4 mo for nonmethylated patients. The median progression-free survival for MGMT-methylated patients in the glioblastoma and gliosarcoma cohorts was 9.1 mo (event rate, 44%) and 13.6 mo (event rate, 50%), respectively, whereas for nonmethylated patients, it was 6.8 mo (event rate, 78%) and 6.2 mo (event rate, 72%) (Supplemental Fig. 2).
(A and B) Median overall survival (OS) and progression-free survival (PFS) were 9.2 and 7.2 mo in glioblastoma cohort, respectively. (C and D) In gliosarcoma cohort, median overall survival and progression-free survival were 8.4 and 6.2 mo, respectively.
FAP Uptake and Its Correlation with FAP Score
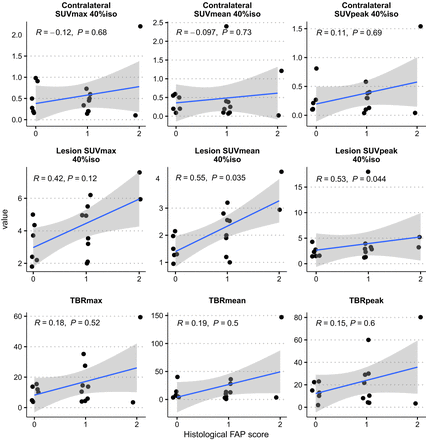
We analyzed the correlation between FAP uptake in 68Ga-FAPI-46 PET scans and FAP expression in tissue samples from 15 glioblastoma patients with preoperative MRI indications of malignant gliomas (Fig. 4). No patients from the gliosarcoma group underwent these scans. The results showed enhanced FAP uptake in the lesions, with a mean SUVmax (40% isocontour) of 4.24 ± 1.69, an SUVmean (40% isocontour) of 2.18 ± 0.91, and an SUVpeak (40% isocontour) of 3.58 ± 4.14, compared with significantly lower values in the contralateral side (SUVmax, 0.55 ± 0.54; SUVmean, 0.44 ± 0.62; SUVpeak, 0.35 ± 0.39). A significant positive correlation was found between tissue-based FAP expression and the lesions’ SUVmean (r = 0.55, P = 0.035) and SUVpeak (r = 0.53, P = 0.044), suggesting FAP’s potential as a biomarker in PET imaging. However, no significant correlation was observed between the lesions’ SUVmax (r = 0.42, P = 0.12) or the contralateral SUVmax (r = −0.12, P = 0.68) and tissue FAP expression. Additionally, maximum TBR (r = 0.18, P = 0.52), mean TBR (r = 0.19; P = 0.5), and peak TBR (r = 0.15; P = 0.6) did not show significant correlations, highlighting the limitations of the maximum TBR as a reliable indicator in 68Ga-FAPI-46 PET scans due to low background uptake and variable interindividual contrast (Fig. 5).
T1-weighted (T1w) contrast-enhanced MRI and 68Ga-FAPI-46 PET (SUV, 0–6) of patients with glioblastoma (isocitrate dehydrogenase wild type) with FAP score of 2 and SUVmax of 7.6 (A), patient with glioblastoma (isocitrate dehydrogenase wild type) with FAP score of 1 and SUVmax of 3.5 (B), and patient with glioblastoma (isocitrate dehydrogenase wild type) with negative FAP score and SUVmax of 2.2 (C). Postprocess coregistration of MRI and 68Ga-FAPI-46 was performed in all cases. HISTO = histopathology; IHC = immunohistochemical.
Lesion SUVmean (r = 0.55, P = 0.035) and SUVpeak (r = 0.53; P = 0.044) show significant positive correlation between FAP uptake in PET and FAP expression in tissues. iso = isocontour; TBRmax = maximum tumor-to-brain ratio; TBRmean = mean tumor-to-brain ratio; TBRpeak = peak tumor-to-brain ratio.
DISCUSSION
Our study, to our knowledge, is the largest to have data on immunohistochemical FAP staining in gliosarcoma and glioblastoma patients with poor survival prognosis. The study shows FAP expression is more pronounced in gliosarcoma than in glioblastoma. In our glioblastoma cohort, SUVmean and SUVpeak in 68Ga-FAPI-46 PET correlated with immunohistochemical FAP expression. The FAP expression amount in 68Ga-FAPI-46 PET depends on cancer type, with sarcoma, esophageal cancer, breast cancer, cholangiocarcinoma, and lung cancer showing a high SUVmax, whereas pheochromocytoma, renal cell cancer, differentiated thyroid cancer, adenoid cystic cancer, and gastric cancer exhibit lower FAP uptake (27). There is a known positive correlation between 68Ga-FAPI-46 SUVmax and the immunohistochemical FAP score in sarcoma (28). FAP PET quantifies the amount of FAP in tumors. Gross tumor volumes are shown to be larger with FAP PET plus MRI than with MRI alone in glioblastoma (29).
No correlation was found between progression-free or overall survival and FAP expression in our glioblastoma cohort, but FAP is a promising theranostic target in high-risk, poor-prognosis gliosarcoma. FAP has radiopharmaceutical therapy potential in xenografts (30). The correlation between SUV and tissue FAP expression suggests that 68Ga-FAPI-46 PET could identify candidates who will respond to radiolabeled FAP therapy.
FAP’s prognostic value remains unclear. Higher expression often correlates with aggressive tumors, but some studies show better prognosis. Higher FAP expression is associated with poorer glioblastoma survival (10), better breast cancer prognosis but poorer colon cancer prognosis (31,32), and decreased metastatic colon cancer survival (26). Positive correlations exist in osteosarcoma between higher FAP expression with advanced clinical stage, high histological grade, positive metastatic status, and shorter survival (33). In gastric cancer, higher FAP expression correlates with poorer differentiation, tumor stage, and invasion (34). FAP is highly expressed in pancreatic adenocarcinoma. Higher peritumoral expression increased the node positivity, recurrence, and death risk (35).
Interestingly, 3 additional grade 4 isocitrate dehydrogenase–mutated astrocytomas scored 0 for immunohistochemical FAP expression in our trial (data not shown). Isocitrate dehydrogenase mutation confers improved glioma survival (36). There is no elevated 68Ga-FAPI-46 PET uptake in isocitrate dehydrogenase–mutant gliomas, allowing noninvasive differentiation from high-grade gliomas (20).
FAP-positive tumor cells correlate with glioblastoma neoangiogenesis, implicating FAP in disease progression (25), fitting our observation of perivascular FAP expression.
FAP antibody expression attenuated HT29 xenograft growth, indicating therapeutic potential (37). However, the clinical FAP inhibitor talabostat and FAP antibody sibrotuzumab trials showed minimal metastatic disease efficacy (38–41). Earlier administration might improve the results.
Our study has several limitations. As a retrospective analysis, we could not control potential confounding factors that may have influenced survival outcomes. The sample size, particularly for gliosarcoma patients, was relatively small. Our study was restricted to tissue samples from deceased patients who survived less than 2.5 y, as per our ethical approval. Consequently, we cannot extrapolate the prognostic value of FAP to all glioblastoma or gliosarcoma patients. A broader, representative sample or a prospective study would be needed to assess FAP’s prognostic value across a more diverse patient cohort, a direction we find promising based on our current findings. We used immunohistochemistry to evaluate FAP expression, but including additional methods such polymerase chain reaction could provide more quantitative results. Regrettably, our cohort did not include patients with gliosarcoma who underwent 68Ga-FAPI-46 PET imaging before their initial surgery. As a result, our analysis was limited to demonstrating the correlation between FAP expression in tissue samples and its uptake in PET scans, specifically in glioblastomas that lacked sarcomatous differentiation. We can draw associative conclusions only regarding FAP’s role in prognosis and treatment response. The single-institution design may limit the generalizability of the findings. Further prospective research in more extensive, multicenter cohorts is warranted to validate the relationships suggested by our preliminary data and to determine the utility of FAP-directed therapies.
CONCLUSION
Our findings reveal greater FAP expression in gliosarcoma than in glioblastoma, suggesting potential therapeutic implications warranting further investigation. The correlation between FAP tissue expression and SUV in 68Ga-FAPI-46 PET for glioblastoma without sarcomatous differentiation allows noninvasive identification of suitable patient populations for FAP radiopharmaceutical therapy by imaging. The PET–tissue staining correlation provides a noninvasive means to identify patients most likely to benefit from FAP-targeted treatment. However, additional studies are needed to clarify FAP’s conflicting prognostic roles across cancer types.
DISCLOSURE
Christoph Oster has received travel support from Novocure and honoraria from Horizon and Novocure. He has received a Clinician Scientist Stipend of the University Medicine Essen Clinician Scientist Academy (UMEA) sponsored by the faculty of medicine and Deutsche Forschungsgemeinschaft (DFG). Kim Pabst has received a Clinician Scientist Stipend of the University Medicine Essen Clinician Scientist Academy (UMEA) sponsored by the faculty of medicine and Deutsche Forschungsgemeinschaft (DFG), travel fees from IPSEN, and research funding from Bayer and is a consultant for Novartis. Teresa Schmidt received honoraria and travel support from Novocure. Lazaros Lazaridis received honoraria and travel support from Novocure. Björn Scheffler is supported by the German Cancer Consortium (DKTK). Sied Kebir received honoraria and travel support from Novocure. Ken Herrmann reports receiving consultant fees from Advanced Accelerator Applications, a Novartis company, Amgen, AstraZeneca, Bain Capital, Bayer, Boston Scientific, Convergent, Curium, Debiopharm, EcoR1, Fusion, GE Healthcare, Immedica, Isotopen Technologien München, Janssen, Merck, Molecular Partners, NVision, POINT Biopharma, Pfizer, Radiopharm Theranostics, Rhine Pharma, Siemens Healthineers, SOFIE Biosciences, Telix, Theragnostics, and Ymabs; receiving research grants from Advanced Accelerator Applications, a Novartis company, Boston Scientific, and Janssen; and having stock or other ownership interests with AdvanCell, Aktis Oncology, Convergent, NVision, Pharma 15, and SOFIE Biosciences. Martin Glas has received research grants from Novocure. He has received honoraria from Roche, Seagan, Servier, Novartis, UCB, Abbvie, Daiichi Sankyo, Bayer, Janssen-Cilag, Kyowa Kirin, Medac, Merck, and Novocure. He has received travel support from Novocure and Medac. No other potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: How does FAP expression in gliosarcoma and glioblastoma correlate with 68Ga-FAPI-46 PET uptake?
PERTINENT FINDINGS: Analysis showed a significant correlation between immunohistochemical FAP expression and SUVmean and SUVpeak in 68Ga-FAPI-46 PET, indicating FAP expression in the tissue.
IMPLICATIONS FOR PATIENT CARE: Correlation between PET imaging and tissue staining provides a noninvasive means to identify patients most likely to benefit from FAP-targeted therapy.
Footnotes
↵* Contributed equally to this work.
Published online Jul. 3, 2024.
- © 2024 by the Society of Nuclear Medicine and Molecular Imaging.
REFERENCES
- Received for publication December 2, 2023.
- Accepted for publication May 22, 2024.