Visual Abstract
Abstract
This study aimed to evaluate the prognostic value of 18F-FDG PET/CT qualitative assessment in terms of recurrence-free survival (RFS), colostomy-free survival (CFS), and overall survival (OS) after radiation therapy (RT) of squamous cell carcinoma of the anus (SCCA). Secondary objectives were to evaluate the prognostic value of baseline and posttherapeutic quantitative 18F-FDG PET/CT parameters in terms of RFS, CFS, and OS. Methods: We included all consecutive patients from the French multicentric cohort FFCD-ANABASE who had undergone 18F-FDG PET/CT at baseline and 4–6 mo after RT or chemoradiotherapy for a localized SCCA. Qualitative assessments separated patients with complete metabolic response (CMR) and non-CMR. Quantitative parameters were measured on baseline and posttreatment 18F-FDG PET/CT. RFS, CFS, and OS were analyzed using the Kaplan–Meier method. Associations among qualitative assessments, quantitative parameters, and RFS, CFS, and OS were analyzed using univariate and multivariate Cox regression. Results: Among 1,015 patients treated between January 2015 and April 2020, 388 patients (300 women and 88 men) from 36 centers had undergone 18F-FDG PET/CT at diagnosis and after treatment. The median age was 65 y (range, 32–90 y); 147 patients (37.9%) had an early-stage tumor and 241 patients (62.1%) had a locally advanced-stage tumor; 59 patients (15.2%) received RT, and 329 (84.8%) received chemoradiotherapy. The median follow-up was 35.5 mo (95% CI, 32.8–36.6 mo). Patients with CMR had better 3-y RFS, CFS, and OS, at 84.2% (95% CI, 77.8%–88.9%), 84.7% (95% CI, 77.2%–89.3%), and 88.6% (95% CI, 82.5%–92.7%), respectively, than did non-CMR patients, at 42.1% (95% CI, 33.4%–50.6%), 47.9% (95% CI, 38.1%–56.8%), and 63.5 (95% CI, 53.2%–72.1%), respectively (P < 0.0001). Quantitative parameters were available for 154 patients from 3 centers. The following parameters were statistically significantly associated with 3-y RFS: baseline SUVmax (primitive tumor [T]) (hazard ratio [HR], 1.05 [95% CI, 1.01–1.1; P = 0.018]), SUVpeak (T) (HR, 1.09 [95% CI, 1.02–1.15; P = 0.007]), MTV 41% (T) (HR, 1.02 [95% CI, 1–1.03; P = 0.023]), MTV 41% (lymph node [N]) (HR, 1.06 [95% CI, 1.03–1.1; P < 0.001]), MTV 41% (T + N) (HR, 1.02 [95% CI, 1–1.03; P = 0.005]), and posttreatment SUVmax (HR, 1.21 [95% CI, 1.09–1.34; P < 0.001]). Conclusion: Treatment response assessed by 18F-FDG PET/CT after RT for SCCA has a significant prognostic value.18F-FDG PET/CT could be useful for adapting follow-up, especially for patients with locally advanced-stage tumors. Quantitative parameters could permit identification of patients with a worse prognosis but should be evaluated in further trials.
Squamous cell carcinoma of the anus (SCCA) is considered a rare tumor, accounting for about 2,000 new cases per year in France (1). Its incidence is rising, but the age at diagnosis is decreasing, allowing for an earlier diagnosis, mostly at a localized stage. Only 5% of cases are diagnosed at a metastatic stage (2).
The standard of care for patients with localized disease is radiation therapy (RT) associated with chemotherapy, including mitomycin C and 5-fluorouracil with curative intent (3). Surgery is a salvage treatment in cases of locoregional relapse.
18F-FDG PET/CT is recommended for the initial staging of SCCA in the French guidelines (4,5) and is considered an option by the European Society of Medical Oncology (6). Indeed, prospective and retrospective studies have shown good performance for 18F-FDG PET/CT, especially in lymph node staging (7,8), modifying the TNM classification in 15%–40% of cases (9,10). Thus, identifying pathologic lymph nodes can modify the RT plan and can be useful for target volume delineation (11,12). Moreover, some metabolic parameters measured by baseline 18F-FDG PET/CT, such as metabolic tumor volume (MTV) or total lesion glycolysis (TLG), could have prognostic value (13–16). Studying these parameters could allow identification of patients with a high risk of relapse or treatment failure. During follow-up after treatment, the role of 18F-FDG PET/CT is not clearly defined. 18F-FDG PET/CT is recommended when relapse is suspected (4) but could also be useful to assess treatment response.
This study aimed to evaluate the prognostic value of 18F-FDG PET/CT assessment in terms of recurrence-free survival (RFS), colostomy-free survival (CFS), and overall survival (OS) after RT of SCCA. We studied the prognostic value of qualitative response on 18F-FDG PET/CT performed 4–6 mo after RT or chemoradiotherapy, and we identified prognostic factors among quantitative parameters measured on 18F-FDG PET/CT.
MATERIALS AND METHODS
Patients treated for SCCA between January 2015 and April 2020 were included in the cohort for French Federation of Digestive Oncology (FFCD)-ANABASE, which is a prospective multicentric observational study conducted by the FFCD. This study aimed to evaluate clinical practice, treatments, and oncologic outcomes for SCCA in France, and the main results have been published (17). The ethics committee (CCTIRS-15.698) and the Commission National de l’Informatique et des Libertés (authorization 915622) approved this retrospective study, and the requirement to obtain written informed consent was waived. All patients received written information and provided oral informed consent.
Among the patients included in the FFCD-ANABASE cohort, we focused in this study on those who had undergone 18F-FDG PET/CT at baseline and again at 4–6 mo after the end of RT or chemoradiotherapy. The main objectives were to evaluate the prognostic value of 18F-FDG PET/CT qualitative response to treatment in terms of RFS, CFS, and OS. Secondary objectives were to identify prognostic factors among quantitative parameters measured on baseline and posttreatment 18F-FDG PET/CT in terms of RFS, CFS, and OS.
Image Acquisition and Interpretation
The following data were collected prospectively and entered into the database by the physicians of each center: SUVmax and presence of significant 18F-FDG uptake for baseline 18F-FDG PET/CT, and SUVmax and global qualitative evaluation for posttreatment 18F-FDG PET/CT.
A complete metabolic response (CMR) was defined as the visual absence of residual 18F-FDG uptake or the presence of nonpathologic minimal residual uptake (left at the discretion of each nuclear medicine physician). A partial metabolic response was defined as any persistent pathologic uptake in the lesions visible on the baseline image. Stability was defined as findings similar to those on the baseline scan. Progressive disease was defined as an increase in uptake because of tumor growth or new pathologic uptake because of the development of a new site of disease.
Moreover, we decided to further analyze the 18F-FDG PET/CT data of patients from 3 large inclusion centers accredited by European Association Research Ltd., which is an accreditation program developed in collaboration with the European Organization for Research and Treatment of Cancer with the aim of providing a common standard for harmonizing the acquisition and interpretation of PET/CT.
Quantitative 18F-FDG PET/CT parameters were collected retrospectively by 2 pairs of physicians (an RT resident and a nuclear medicine senior) by reviewing the native 18F-FDG PET/CT images. These parameters were measured using a volume of interest placed by the physicians over the primary tumor and each involved lymph node. SUVmax and SUVpeak were, respectively, defined as the maximum voxel intensity and the average SUV within a 1 cm3 volume of interest centered on the hottest area of the tumor or lymph node. Metabolic tumor volume (MTV) 41% was defined as the hypermetabolic tissue volume with a cutoff greater than 41% of SUVmax. SUVmean was defined as the mean of SUV of all voxels within the MTV.
The following data were collected on baseline 18F-FDG PET/CT (where T indicates primitive tumor and N indicates lymph nodes): SUVmax (T), SUVpeak (T), SUVmean (T), and MTV 41% (T). Total lesion glycolysis (TLG) (T) was calculated (SUVmean [T] × MTV 41% [T]). MTV 41% (N) and SUVmean (N) were collected for zero to 10 lymph nodes. TLG (N) was calculated for each lymph node (SUVmean [N] × MTV 41% [N]). Sums were realized to obtain MTV 41% ([total] N), TLG ([total] N), MTV 41% (T + N), and TLG (T + N).
A quantitative evaluation was realized on posttreatment 18F-FDG PET/CT with a measure of posttreatment SUVmax, allowing calculation of change in SUVmax ([pretreatment SUVmax – posttreatment SUVmax]/pretreatment SUVmax × 100).
Statistical Analysis
RFS was defined as the time between the start of treatment and the first recurrence or death (from any cause). CFS was defined as the time between the start of treatment and the first colostomy or death (from any cause). Alive patients without recurrence or colostomy were censored at the date of the last follow-up. OS was defined as the time between the start of treatment and death (from any cause). Alive patients were censored at the date of the last follow-up.
Descriptive analyses were performed for each 18F-FDG PET/CT parameter. RFS, CFS, and OS were analyzed using the Kaplan–Meier method and described using medians with 2-sided 95% CIs. Log-rank tests were used to compare rates and event-time distributions with a 95% CI. Univariate and multivariate analyses were done to evaluate the association between qualitative response to treatment on 18F-FDG PET/CT; other parameters linked to 18F-FDG PET/CT and clinical parameters; and RFS, CFS, and OS using Cox proportional hazards regression reporting hazard ratios (HRs) and 95% CI. A receiver operating characteristic curve was used to determine a discriminative threshold value of posttreatment SUVmax in terms of RFS, CFS, and OS.
RESULTS
Patient Characteristics
Among 1,015 patients who received first-line RT or chemoradiotherapy for nonmetastatic SCCA between January 2015 and April 2020, 388 from 36 centers underwent 18F-FDG PET/CT at baseline and 4–6 mo after treatment (Fig. 1). There were 88 (22.7%) men and 300 (77.3%) women. The median age was 64 y (range, 32–90 y). Patient and tumor characteristics are presented in Table 1.
Flowchart. CRT = chemoradiotherapy.
Patient and Tumor Characteristics
Fifty-nine patients (15.2%) received RT, and 329 (84.8%) received chemoradiotherapy, with concurrent mitomycin-5-fluorouracil for 286 patients (86.9%) and cisplatin-5-fluorouracil for 14 patients (4.3%). The median RT dose was 60 Gy on the tumor volume and 45 Gy on the pelvis. Among patients previously described, 154 patients from 3 main recruiter centers had a secondary analysis with quantitative evaluation of baseline and posttreatment 18F-FDG PET/CT.
Outcomes
Median follow-up was 35.5 mo (95% CI, 32.8–36.6). The 3-y RFS, CFS, and OS for the whole population were 68.0% (95% CI, 62.5–72.9), 70.5% (95% CI, 64.8–75.5), and 79.2% (95% CI, 73.8–83.7), respectively. Among the 242 patients with CMR, 213 (88%) were free of recurrence at 3 y. Among the 146 patients with non-CMR, 77 (52.7%) had a recurrence at 3 y.
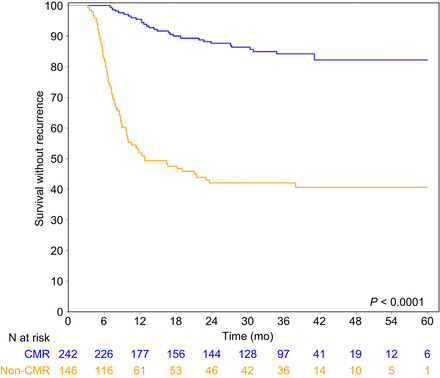
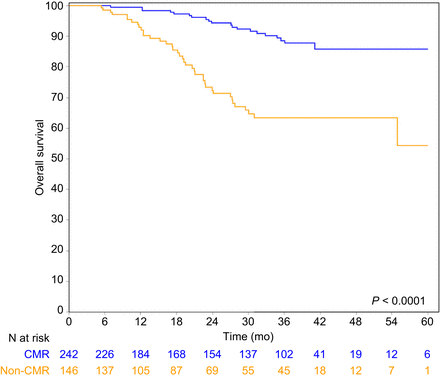
The 3-y RFS was 84.2% (95% CI, 77.8–88.9) for patients with CMR, compared with 42.1% (95% CI, 33.4–50.6) for patients without CMR (P < 0.0001) (Fig. 2). Similarly, the 3-y CFS was 84.7% (95% CI, 78.2–89.3) for patients with CMR and 47.9% (95% CI, 38.1–56.8) for patients without CMR (P < 0.0001) (Fig. 3). The 3-y OS was 88.6% (95% CI, 82.5–92.7) for patients with CMR and 63.5 (95% CI, 53.2–72.1) for patients without CMR (P < 0.0001) (Fig. 4).
RFS curves of CMR patients and non-CMR patients.
CFS curves of CMR patients and non-CMR patients.
OS curves of CMR patients and non-CMR patients.
Qualitative response to treatment on 18F-FDG PET/CT was statistically significantly associated with better RFS, CFS, and OS on both univariate and multivariate analysis (Table 2). A descriptive analysis of quantitative 18F-FDG PET/CT parameters analyzed on 154 patients is presented in Table 3.
Association Between 18F-FDG PET/CT Qualitative Treatment Response and RFS, CFS, and OS
Descriptive Analysis of 18F-FDG PET/CT Parameters
The results of univariate analysis between 18F-FDG PET/CT parameters and RFS, CFS, and OS are presented in Table 4. An increase of 1 unit of baseline SUVmax (T), SUVpeak (T), MTV 41% (T), MTV 41% (N), MTV 41% (T + N), and posttreatment SUVmax was significantly associated with a poor RFS, CFS, and OS. There was no statistically significant prognostic impact of TLG and change in SUVmax.
Association Between 18F-FDG PET/CT Parameters and OS, RFS, and CFS (Univariate Analysis)
By using a receiver operating characteristic curve, we found that a threshold of 5 for posttreatment SUVmax separates patients into prognostic groups. The recurrence rate was 35% for patients with a posttreatment SUVmax of more than 5 and 18.4% for patients with a posttreatment SUVmax 5 or less (HR, 0.44 [95% CI, 0.22–0.87]; P = 0.018). Similarly, the colostomy rate was 35% for patients with a posttreatment SUVmax of more than 5 and 14.68% for patients with a posttreatment SUVmax of 5 or less (HR, 0.30 [95% CI, 0.14–0.61]; P = 0.001). OS did not significantly differ between these 2 groups (HR, 0.47 [95% CI, 0.2–1.08]; P = 0.075).
DISCUSSION
The purpose of this study was to determine the prognostic value of posttreatment 18F-FDG PET/CT in patients treated with RT or chemoradiotherapy for nonmetastatic SCCA. To our knowledge, our study, with a population of 388 patients, is one of the largest that aimed to assess the predictive value of 18F-FDG PET/CT response to treatment. We confirmed the significant prognostic value of 18F-FDG PET/CT qualitative response to treatment in terms of RFS, CFS, and OS.
Several studies have previously examined the value of treatment response assessed by 18F-FDG PET/CT and showed that a CMR is highly associated with better progression-free survival, OS (18,19), and cause-specific survival (20). Interestingly, metabolic response to treatment has even been found to be a more significant predictor factor of progression-free survival than pretreatment tumor size (based on physical examination) and nodal status in a study of 53 patients (21). Finally, it has also been shown that posttreatment 18F-FDG PET/CT has a high negative predictive value and could be used to rule out residual or recurrent disease (22).
Regarding quantitative 18F-FDG PET/CT parameters, we identified several significant prognostic factors: MTV, pretreatment SUVpeak and SUVmax, and posttreatment SUVmax. These results are consistent with literature regarding MTV, assessed in 6 different studies (13–16,23,24), but also regarding pretreatment SUVpeak and posttreatment SUVmax, which have not been frequently assessed (16,25). Literature regarding pretreatment SUVmax showed more conflicting results, with a study of 77 patients showing its prognostic value (26) but also studies showing negative results (13,23,24,27).
By using thresholds to separate patients into prognostic groups, we found that a posttreatment SUVmax of 5 or less was predictive of better RFS. A posttreatment SUVmax of less than 6.1 has already been shown to be associated with reduced local recurrence and increased OS (25). In the literature, an MTV 35% threshold at 40 cm3 was shown to be the best cutoff to discriminate a low from a high risk of recurrence (15).
In this study, we have shown 18F-FDG PET/CT to have major prognostic value regarding qualitative treatment response. Even if qualitative evaluation is subjective and is physician-dependent, this study still proves its reliability. Moreover, this study included patients from 36 centers in France with as many physicians, showing reproducibility and confidence in this evaluation.
Finally, we have shown that posttreatment SUVmax was significantly associated with RFS, CFS, and OS. It is the main parameter used in 18F-FDG PET/CT interpretation and analysis and is easy to measure.
Our study had some limitations. Patients were included from 36 centers, potentially leading to heterogeneity in patient management and 18F-FDG PET/CT assessment. The 36 centers could have different 18F-FDG PET/CT equipment. Assessment of CMR was left to the discretion of the nuclear medicine physician of each center. We selected patients with 18F-FDG PET/CT at baseline and 4–6 mo after treatment, but all centers did not have the same follow-up policy after RT or chemoradiotherapy of SCCA. 18F-FDG PET/CT could have been done systematically 4–6 mo after treatment or only when relapse was suspected. Concerning the quantitative parameter study, 18F-FDG PET/CT was performed at 3 different centers, and different PET/CT scanners can have variable quantification of 18F-FDG uptake. Moreover, the images were reviewed retrospectively by 2 physicians, and the analysis was univariate.
Currently, 18F-FDG PET/CT is recommended in cases of relapse or suspicion of treatment failure (4). By showing the major prognostic value of treatment response as assessed by 18F-FDG PET/CT, this study encourages a systematic evaluation by 18F-FDG PET/CT. We know that patients with early-stage tumors (T1–2, N0) and patients with locally advanced-stage tumors (T3–4 or N+) have different prognoses. Disease-free survival at 3 y is around 85% for patients with early-stage SCCA but 66% for patients with locally advanced SCCA (17,28). The 3-y CFS and OS are 86% and 92%, respectively, in the early-stage group compared with 67% and 78% in the locally advanced group (17). Present research about SCCA focuses on more personalized treatment and management according to tumoral stages. Modalities of evaluation and follow-up after treatment could be adapted too. Patients with early-stage tumors have a low risk of local or metastatic relapse. Most relapses are local and can be detected by clinical evaluation. Surveillance can rely on clinical examination, which seems to be reliable, whereas 18F-FDG PET/CT could be useful in suspected recurrence. On the other hand, patients with locally advanced-stage tumors still present a poor prognosis with a high risk of local and distant recurrence. Moreover, locally advanced tumors frequently involve adjacent organs or deep lymph nodes that cannot be accurately assessed by physical evaluation.
During follow-up, an evaluation by thoracoabdominopelvic CT is recommended once a year during the first 3 y according to the French and European guidelines (4,6). Pelvic MRI is recommended before salvage surgery (4). Despite past studies showing its value, 18F-FDG PET/CT is currently not included in guidelines for systematic follow-up of patients. By confirming its importance in this large-scale study, we suggest that 18F-FDG PET/CT could be recommended at 4–6 mo after the end of chemoradiotherapy for patients with locally advanced-stage tumors. Modalities of follow-up could be adapted according to the response on 18F-FDG PET/CT, since it is known that a CMR is highly predictive of a good outcome.
CONCLUSION
Metabolic treatment response assessed by 18F-FDG PET/CT after RT or chemoradiotherapy for nonmetastatic SCCA has significant prognostic value in terms of RFS, CFS, and OS. 18F-FDG PET/CT could be useful to assess treatment response and adapt follow-up, especially for patients with locally advanced-stage tumors. Quantitative parameters measured on 18F-FDG PET/CT could permit identification of patients with the worst prognosis but should be evaluated in further trials.
DISCLOSURE
Financial support was received from FFCD. No other potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: Could PET/CT be useful in assessing treatment response after RT of SCCA?
PERTINENT FINDINGS: This prospective cohort study showed PET/CT to have statistically significant prognostic value in assessing treatment response in terms of RFS, CFS, and OS.
IMPLICATIONS FOR PATIENT CARE: PET/CT could be useful to assess treatment response and to adapt follow-up, especially for patients with locally advanced-stage tumors.
Footnotes
Published online Jun. 27, 2024.
- © 2024 by the Society of Nuclear Medicine and Molecular Imaging.
REFERENCES
- Received for publication February 20, 2024.
- Accepted for publication May 28, 2024.