Visual Abstract
Abstract
Currently, cutoffs of quantitative [15O]H2O PET to detect fractional flow reserve (FFR)–defined coronary artery disease (CAD) were derived from a single cohort that included patients without prior CAD. However, prior CAD, sex, and age can influence myocardial blood flow (MBF). Therefore, the present study determined the influence of prior CAD, sex, and age on optimal cutoffs of hyperemic MBF (hMBF) and coronary flow reserve (CFR) and evaluated whether cutoff optimization enhanced diagnostic performance of quantitative [15O]H2O PET against an FFR reference standard. Methods: Patients with chronic coronary symptoms underwent [15O]H2O PET and invasive coronary angiography with FFR. Optimal cutoffs for patients with and without prior CAD and subpopulations based on sex and age were determined. Results: This multicenter study included 560 patients. Optimal cutoffs were similar for patients with (n = 186) and without prior CAD (hMBF, 2.3 vs. 2.3 mL·min−1·g−1; CFR, 2.7 vs. 2.6). Females (n = 190) had higher hMBF cutoffs than males (2.8 vs. 2.3 mL·min−1·g−1), whereas CFRs were comparable (2.6 vs. 2.7). However, female sex–specific hMBF cutoff implementation decreased diagnostic accuracy as compared with the cutoff of 2.3 mL·min−1·g−1 (72% vs. 82%, P < 0.001). Patients aged more than 70 y (n = 79) had lower hMBF (1.7 mL·min−1·g−1) and CFR (2.3) cutoffs than did patients aged 50 y or less, 51–60 y, and 61–70 y (hMBF, 2.3–2.4 mL·min−1·g−1; CFR, 2.7). Age-specific cutoffs in patients aged more than 70 y yielded comparable accuracy to the previously established cutoffs (hMBF, 72% vs. 76%, P = 0.664; CFR, 80% vs. 75%, P = 0.289). Conclusion: Patients with and without prior CAD had similar [15O]H2O PET cutoffs for detecting FFR-defined significant CAD. Stratifying patients according to sex and age led to different optimal cutoffs; however, these values did not translate into an increased overall accuracy as compared with previously established thresholds for MBF.
- PET
- fractional flow reserve
- cutoff values
- hyperemic myocardial blood flow
- coronary flow reserve
- coronary artery disease
A wide array of diagnostic modalities is available to evaluate the presence and functional repercussion of coronary artery disease (CAD). Among these diagnostic tools, [15O]H2O PET is considered the gold standard for noninvasive quantification of myocardial blood flow (MBF) and has well-established diagnostic and prognostic value (1–3). PET serves as a gatekeeper for invasive coronary angiography (ICA), where fractional flow reserve (FFR) is used to guide revascularization, considering its prognostic importance (4,5). One of the challenges of quantitative MBF interpretation to guide revascularization decision-making is the definition of normal and abnormal MBF, paralleling significant and nonsignificant FFR measurements. Currently used [15O]H2O PET cutoffs were derived from a collaborative study that found a cutoff of 2.3 mL·min−1·g−1 for optimal hyperemic MBF (hMBF) and 2.5 for coronary flow reserve (CFR) to detect FFR-defined CAD (6). These cutoffs were established in a population without prior CAD, whereas contemporary guidelines endorse myocardial perfusion imaging for patients with a substantial clinical likelihood of obstructive CAD, including those with a prior CAD history (7,8). Patients with prior CAD and recurrence of symptoms have more advanced atherosclerotic disease and a higher prevalence of microvascular dysfunction, which can cause diminished MBF even in the absence of obstructive epicardial lesions (9,10). Moreover, prior studies revealed that both sex and age can affect myocardial perfusion values, irrespective of the presence of obstructive CAD (6,11–16). Conceivably, recalibrating [15O]H2O PET thresholds for the presence of prior CAD, sex, and age could enhance diagnostic performance in these distinct subpopulations. Therefore, the aim of this study was to assess how prior CAD, sex, and age impact the optimal cutoffs of quantitative [15O]H2O PET in detecting FFR-defined hemodynamically significant CAD. We further sought to compare the diagnostic performance of [15O]H2O PET using both general and patient-specific cutoffs.
MATERIALS AND METHODS
Patient Population
This was a multicenter study that included patients suspected of having obstructive CAD who underwent [15O]H2O PET perfusion imaging and ICA in conjunction with FFR measurements. The patient population was derived from 3 participating centers: Amsterdam University Medical Center (n = 439), Turku University Hospital (n = 116), and Uppsala University Hospital (n = 5). Patients from the Amsterdam University Medical Center were derived from a clinical registry (n = 49), from the PACIFIC-1 trial (n = 204), or from the PACIFIC-2 trial (n = 186) (9,10). Patients from Turku University Hospital and Uppsala University Hospital were derived from clinical registries. General exclusion criteria were atrial fibrillation, contraindications for adenosine, pregnancy, and a history of coronary artery bypass graft surgery. Additional specific exclusion criteria of the PACIFIC trials are described elsewhere (9,10). Patients from the PACIFIC-2 trial had a documented cardiac history of myocardial infarction (MI) and/or percutaneous coronary intervention (PCI), whereas these patients were excluded from the PACIFIC-1 trial and clinical registries. Furthermore, patients from the PACIFIC-1 trial and clinical registries had a left ventricular ejection fraction of more than 50% on echocardiography. The maximum allowed interval between PET and ICA was 3 mo, and no cardiac events were documented within this period. Each center had the approval of a local institutional ethical review board, and all patients provided written informed consent.
[15O]H2O PET Perfusion Imaging, Quantification of MBF, and Interpretation of Perfusion Defect
All patients underwent [15O]H2O PET perfusion imaging using a hybrid PET/CT scanner with site-specific protocols. These protocols and quantification of MBF are described in the supplemental materials (available at http://jnm.snmjournals.org) (6,17–21). PET perfusion imaging polar maps were visually assessed for presence of a perfusion defect, defined as a defect of 2 or more adjacent segments within the right coronary artery territory and circumflex artery territory, and 4 or more adjacent segments within the left anterior descending artery territory. In the absence of a visual perfusion defect, the mean vascular hMBF/CFR was used for further analysis. If a visual perfusion defect was present, the mean hMBF/CFR of the involved segments was used for further analysis. Overlapping segments of a perfusion defect were not included in the mean perfusion value of the adjacent vascular territory. An example of the interpretation of a visual perfusion defect is shown in Supplemental Figure 1. Parametric segmental MBF values were allocated to their corresponding vascular territory according to the American Heart Association 17-segment model after correction for coronary dominance based on information obtained from ICA (21).
Scar Detection
In the context of the PACIFIC-2 trial, all patients with prior CAD included in our study received cardiac MRI besides PET perfusion imaging. For these individuals, the cardiac MRI data served to detect vascular areas with significant scarring. The extent of late gadolinium enhancement was visually analyzed according to the American Heart Association 17-segment model (excluding the apex) using a 5-point scale (0%, 1%–25%, 26%–50%, 51%–75%, >75%) (21). Significant scarring was defined as a vascular late gadolinium enhancement score of 2 or more. An additional description of the cardiac MRI acquisition protocol has been previously published (10).
ICA and FFR
ICA was performed using standard protocols. FFR was calculated by dividing the mean distal intracoronary pressure by the mean arterial pressure after inducing maximal hyperemia by infusion of intracoronary (150 μg) or intravenous (140 μg·kg−1·min−1) adenosine. In the context of the PACIFIC-1 and -2 trials, FFR measurements were performed in all major coronary arteries and side branches larger than 2.0 mm. For all other patients, FFR was measured in intermediate lesions (diameter stenosis, 30%–90%). Hemodynamically significant CAD was defined as an FFR of 0.80 or less or as coronary lesions with a diameter stenosis of more than 90% if FFR measurements were not performed. Conversely, vessels without an FFR of 0.80 or less or coronary lesions with a diameter stenosis of less than 30% were considered non-obstructive. Vessels with intermediate stenosis and no FFR measurement were excluded from further analysis, as were right coronary arteries functioning as a right ventricular branch or coronary anomalies.
Statistical Analysis
Univariable and multivariable regression analysis was performed to identify patient characteristics and traditional CAD risk factors that influence hMBF. The regression analysis was conducted using a linear mixed-effects model to account for multiple vessels deriving from the same patient. Interactions between prior MI, prior PCI, sex, and age were explored. Optimal cutoffs for hMBF and CFR were calculated for specific subpopulations stratified by the presence of prior CAD (defined as a prior MI and/or PCI), sex, and age (≤50, 51–60, 61–70, and >70 y), using the Youden index. Areas under the receiver-operating characteristic curve (AUC) were constructed using hMBF and CFR on both a per-patient (lowest vascular value of a patient) and per-vessel level and were compared using the DeLong method. The diagnostic performance of [15O]H2O PET–derived hMBF and CFR was assessed using both the previously established general cutoffs (hMBF, 2.3 mL·min−1·g−1; CFR, 2.5 (6)) and patient-specific optimal cutoffs for subpopulations in which hMBF cutoffs differed by more than 0.1 mL·min−1·g−1 from the general cutoff. The diagnostic performance of hMBF and CFR using the general cutoffs was calculated as well for all patients from the Amsterdam University Medical Centers and for all other patients. Per-patient diagnostic performance measures (sensitivity, specificity, positive and negative predictive values, and accuracy) were compared using the McNemar or χ2 test (sensitivity, specificity, and accuracy) or using generalized estimation equations with an independent correlation structure (positive and negative predictive values). Per-vessel diagnostic performance measures were compared using generalized estimation equations with an exchangeable (sensitivity, specificity, and accuracy) or independent (positive and negative predictive values) correlation structure, to correct for multiple vessels deriving from the same patient. A 2-sided P value of less than 0.05 was considered statistically significant. Statistical analyses were performed using the SPSS software package (version 28.0; IBM) and MedCalc Statistical Software (version 20.006; MedCalc Software Ltd.).
RESULTS
Study Population
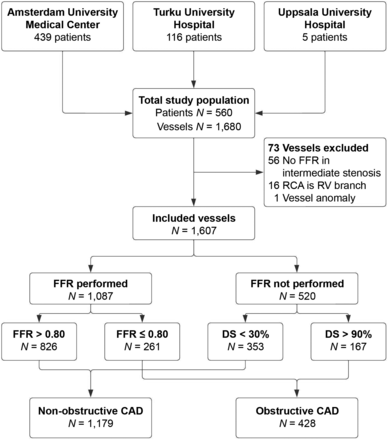
In total, 560 patients were eligible for inclusion in this study. Among their 1,680 vessels, 73 (4%) were excluded from further analysis: 56 (3%) because of the absence of FFR in an intermediate stenosis, 16 (1%) because the right coronary artery was a right ventricular branch, and 1 (<1%) because it was a coronary anomaly. As a result, 560 patients and 1,607 vessels were included (Fig. 1). Baseline characteristics are presented in Table 1. Mean age was 61 ± 9.1 y, and 370 (66%) patients were male. A total of 186 (33%) patients had prior CAD (98 [18%] prior MI and 168 [30%] prior PCI). Supplemental Table 1 presents the baseline characteristics stratified by patients with prior CAD, sex, and age. Of patients with prior CAD, cardiac MRI-defined left ventricular ejection fraction was less than 35% in 5 patients and 35%–54% in 44 patients. All other patients had a normal left ventricular ejection fraction.
Study flowchart of included patients and vessels, with corresponding presence of FFR interrogation. DS = diameter stenosis; RCA = right coronary artery; RV = right ventricular.
Baseline Characteristics of Total Study Population (n = 560)
MBF in Relationship to Prior CAD, Sex, and Age
The relationship between myocardial perfusion and prior CAD, sex, and age in vessels with and without obstructive CAD is depicted in Figure 2. In patients with a prior CAD, hMBF and CFR were lower in non-obstructive vessels but higher in obstructive vessels, when compared to patients without prior CAD. hMBF was higher in females than males for both non-obstructive and obstructive vessels, whereas CFR was comparable between the sexes. Among the age groups 50 y or less, 51–60 y, and 61–70 y, hMBF and CFR exhibited no significant differences. However, patients aged more than 70 y had lower hMBF in non-obstructive vessels than did patients aged 50 y or less or patients aged 51–60 y. Furthermore, CFR in patients aged more than 70 y was lower in both non-obstructive vessels (as compared with all other groups) and obstructive vessels (as compared with patients aged 51–60 y and patients aged 61–70 y). Resting MBF measures are presented in Supplemental Table 2. Table 2 shows the result of a regression analysis describing the influence of patient-specific characteristics and traditional CAD risk factors on hMBF. In a multivariable regression analysis, female sex, age, smoking, diabetes, and hypertension were significantly and independently associated with hMBF. There was a significant interaction between prior MI and the female sex (P = 0.030) and between prior PCI and the male sex (P = 0.006) (Supplemental Table 3).
Relationship between myocardial perfusion and patient characteristics in vessels with and without obstructive CAD. Patients are divided into subpopulations, stratified by prior CAD (defined as prior MI and/or PCI) (A), sex (B), and age (C).
Uni- and Multivariable Regression Analysis of Influence of Patient-Specific Characteristics and Risk Factors on hMBF
Optimal Cutoffs and Diagnostic Performance of [15O]H2O PET
The vessel-specific optimal cutoffs and corresponding AUC analysis of [15O]H2O PET for predicting obstructive CAD in the patient-specific subpopulations are shown in Table 3 and Supplemental Figure 2. Patients without prior CAD had an optimal cutoff of 2.3 mL·min−1·g−1 for hMBF and 2.6 for CFR. The overall presence of prior CAD, prior MI, and prior PCI did not influence cutoffs. However, in vascular territories with late gadolinium enhancement–defined scarring, CFR showed a cutoff of 2.0. Males showed similar thresholds of hMBF (2.3 mL·min−1·g−1) and CFR (2.7), whereas females exhibited a higher threshold for hMBF, at 2.8 mL·min−1·g−1, with a corresponding cutoff of 2.6 for CFR. Regarding age, only patients aged more than 70 y displayed disparate thresholds: 1.7 mL·min−1·g−1 for hMBF and 2.3 for CFR. Cutoffs based on different FFR and angiographic thresholds are shown in Supplemental Tables 4 and 5.
Vessel-Specific Optimal Cutoffs and AUC Analysis of [15O]H2O PET
Figure 3 shows the AUC analyses with corresponding 95% CIs of the patient-specific subpopulations on a per-patient level. The diagnostic performance of hMBF in patients without prior CAD was significantly higher than in patients with prior CAD (AUC, 0.87 [95% CI, 0.85–0.92] vs. 0.80 [95% CI, 0.74–0.86]; P = 0.021), whereas the diagnostic performance of CFR was comparable between these groups (P = 0.069). For the subpopulations based on sex and age, AUC analysis revealed no significant differences between groups for either hMBF or CFR.
AUC analysis and corresponding 95% CI of [15O]H2O PET–derived hMBF and CFR to detect FFR-defined hemodynamically significant CAD for specific subpopulations on per-patient level.
The per-patient diagnostic performance of hMBF and CFR for patients from the Amsterdam University Medical Center (n = 439) compared with those from Turku University Hospital and Uppsala University Hospital (n = 121) is shown in Supplemental Table 6.
Influence of Cutoff Optimization on Diagnostic Performance of [15O]H2O PET in Females and Patients Aged More Than 70 Years
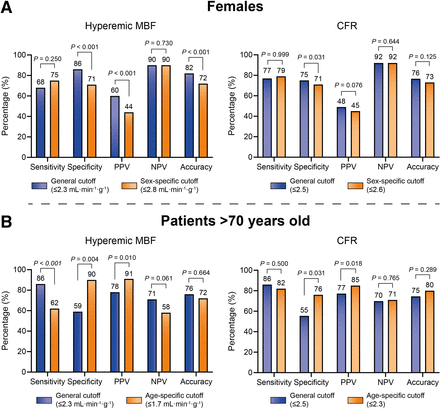
The implementation of optimized cutoffs in females resulted in a decreased per-patient specificity, positive predictive value, and accuracy for hMBF (all P < 0.001), whereas for CFR only specificity decreased (P = 0.031) (Fig. 4A). Per-patient accuracy was highest for hMBF using the general cutoff (82%)—superior to the accuracy of CFR using both the general (76%, P = 0.015) and the specific (73%, P = 0.001) cutoffs. Per-vessel diagnostic performance is shown in Supplemental Table 7. The per-vessel accuracy of hMBF using the general cutoff (87%) was superior to that of hMBF using the specific cutoff (80%, P < 0.001) and to that of CFR using either the general (81%, P < 0.001) or the specific (79%, P < 0.001) cutoff.
Per-patient diagnostic performance of hMBF and CFR with general and patient-specific optimal cutoffs in females (A) and patients > 70 y old (B). NPV = negative predictive value; PPV = positive predictive value.
In patients aged more than 70 y, cutoff optimization led to a reduced per-patient sensitivity for hMBF (P < 0.001), whereas specificity and positive predictive value increased for both hMBF and CFR (all P ≤ 0.031) (Fig. 4B). The highest per-patient accuracy was observed for CFR using the specific cutoff (80%), but there was no statistically significant improvement over the accuracy of hMBF using either the general (76%, P = 0.581) or the specific (72%, P = 0.263) cutoff. Per-vessel diagnostic performance is shown in Supplemental Table 7. Per-vessel accuracy was highest for hMBF using the specific cutoff (83%), outperforming the accuracy of hMBF and CFR using the general cutoffs (both 73%, P = 0.003) and comparable to the accuracy of CFR using the specific cutoff (79%, P = 0.081).
DISCUSSION
This study examined how prior CAD, sex, and age affect the optimal cutoffs of quantitative [15O]H2O PET in identifying FFR-defined hemodynamically significant CAD. Previous efforts have not optimized hMBF and CFR thresholds using [15O]H2O PET for these subgroups. The key findings of this study are that prior MI and/or PCI did not require specific hMBF and CFR thresholds for predicting hemodynamically significant CAD; that females presented a higher hMBF cutoff than did males; that elderly patients (>70 y) exhibited lower thresholds for hMBF and CFR than did younger patients; and that the optimized cutoffs did not improve overall accuracy for these specific subpopulations.
Influence of Prior CAD
Diffuse atherosclerosis and coronary microvascular dysfunction impair myocardial perfusion in patients with prior CAD, resulting in a potential disparity between FFR and MBF when PET is used as a guide for coronary revascularizations (22,23). Moreover, the presence of myocardial scarring influences the amount of viable tissue in a vascular territory, with a subsequent impact on FFR, whereas MBF alterations might occur due to diminished tracer extraction in viable portions of scarred regions (24,25). As a result, the optimal hMBF to distinguish between normal and ischemic MBF as defined by FFR may differ from that of patients without prior CAD. In our study, although perfusion was lower in patients with prior CAD than in their counterparts without prior CAD in non-obstructive vessels, the independent presence of prior MI or PCI did not influence hMBF. Consequently, the optimal cutoffs for hMBF and CFR were consistent with those of patients without prior CAD. Notably, even when vascular territories with the presence of late gadolinium enhancement–defined scarring were considered, optimal hMBF thresholds remained unchanged, although CFR displayed a remarkable decrease in these regions. Prior studies assessing the diagnostic performance of [15O]H2O PET using thresholds similar to those established in our study indicated reduced diagnostic performance in patients with prior CAD compared with those without, which is confirmed by the lower AUC of hMBF in patients with prior CAD than in those without in our study (9,10). Our findings emphasize that these disparities in diagnostic performance are likely related to diffuse and microvascular atherosclerotic disease rather than being the result of suboptimal PET thresholds in either group.
Impact of Sex
Risk factors impact myocardial perfusion irrespective of epicardial atherosclerotic burden by increasing microvascular resistance (26). Sex, however, has been demonstrated to exert the largest influence on stress perfusion values. This apparent phenomenon has been attributed to sex differences in the hormonal milieu and the subsequent protective effects of estrogens in preserving the function of the coronary microvasculature. Indeed, estrogen replacement therapy has been shown to reduce minimal coronary microvascular resistance and thus preserve the functional integrity of the microvasculature (27). An experimental study on animal models, however, has linked blunted resting MBF to testosterone levels, whereas stress perfusion levels remained unchanged (11). Indeed, in a study by Duvernoy et al., hMBF remained significantly higher in females than in their male counterparts despite the fact that the females were postmenopausal (28). Irrespective of the underlying mechanism, one may postulate that a different threshold for myocardial perfusion values should be established to account for the differences in vasomotor function, to optimize the diagnostic value of quantitative PET, and to facilitate its role as a guide for coronary revascularizations. Therefore, females might benefit from a different cutoff for the detection of hemodynamic significant CAD as refereed by FFR, giving an impetus to personalize PET-guided management. In our study, AUCs between sexes were comparable, and specific cutoffs for males did not differ from the prior established thresholds (6). Females, on the other hand, demonstrated higher hMBF cutoffs than did males (2.8 vs. 2.3 mL·min−1·g−1), whereas thresholds for CFR remained comparable between the sexes (2.7 vs. 2.6). Prior studies have shown similar results, although lower CFRs in females have been described as well (6,14,28–30). The threshold for CFR is similar for both males and females, and thus, CFR is preferably used instead of hMBF for large datasets attenuating the bias in flow values introduced by sex on, for example, prediction of adverse events. However, reliance on a higher ischemic threshold in females would result in an overestimation of abnormal stress perfusion scans were it to serve as a gatekeeper test for the catheterization laboratory. Because of the relatively low disease prevalence in females, the increased sensitivity of a higher hMBF cutoff came at the expense of a loss of specificity resulting in an overall reduced accuracy on both a per-vessel and a per-patient level. From a clinical per-patient perspective, the increase in sensitivity was not deemed sufficient to recommend the use of this specific cutoff for minimizing false deferrals to the catheterization laboratory. For CFR, the diagnostic performance of optimized thresholds followed the same trend, albeit not as pronounced given the minimal sex difference for this flow parameter. Accordingly, the accuracy of hMBF surpassed that of CFR despite the implementation of specific CFR thresholds, in line with previous studies (6,31,32). All in all, our results do not support the use of sex-specific cutoffs and indicate that hMBF is the most reliable marker for hemodynamic significant epicardial disease in this specific population.
Effect of Age
As age advances, several factors have been reported to influence myocardial perfusion. Diffuse atherosclerosis causes endothelial dysfunction by prohibiting endothelium-dependent vasodilatation of the coronary arteries. In a study by Egashira et al., an increase in peak coronary blood flow using an endothelium-dependent vasodilator correlated negatively with age, whereas the response to an endothelium-independent vasodilator was only slightly affected by age (33). Moreover, age-related morphologic changes in smooth muscle cells can increase arterial impedance (34). These factors can equally be attributed to the microvasculature, increasing microvascular resistance and subsequently decreasing stress perfusion and flow reserve (15,35). With multiple factors other than epicardial disease reducing myocardial perfusion in elderly patients, correcting perfusion for age might, arguably, optimize diagnosis establishment. In our study, like a study by Uren et al. (15), hMBF and CFR were similar for age groups up to 70 y but declined afterward, whereas AUCs between the groups were comparable. These findings are mirrored by the optimal cutoffs identified for different age groups in our study, where, as age exceeded 70 y, cutoffs decreased to 1.7 mL·min−1·g−1 for hMBF and 2.3 for CFR. With a relative high disease prevalence in older patients, the increase in specificity with age-specific cutoffs outweighed the concomitant fall in sensitivity on a per-vessel level, suggesting a potential benefit from the use of age-specific thresholds. Nevertheless, this difference between the use of cutoffs did not extend to the clinical applicability of perfusion imaging, as reflected by the per-patient analysis. The reduced rate of false positives by lowering the cutoff in the elderly was counterbalanced by the increase in false negatives, resulting in equal diagnostic accuracy. In fact, the conventional cutoff is superior in preventing these patients from a false deferral from the catheterization laboratory. All in all, our findings suggest that the general cutoffs remain applicable even for patients aged more than 70 y.
Role of Cutoffs in Clinical Practice
The use of binary cutoffs seems insurmountable in daily clinical practice, especially when cardiac PET perfusion imaging is being used as a guide for coronary revascularization compared with having a mere gatekeeper function. The difficulty of cutoffs is underscored by a study of Vester et al., in which cutoffs for predicting revascularization and for predicting relief of angina after PCI in patients after coronary artery bypass graft surgery substantially differed from 1.36 to 1.99 mL·min−1·g−1 for hMBF and from 1.20 to 2.35 for CFR (36). Moreover, cutoffs in our study slightly changed when different FFR or angiographic references were applied, further implying that cutoffs depend on the intended clinical purpose. Indeed, any measure of myocardial perfusion is continuous by its biologic nature and cannot merely be confined to a binary threshold. This oversimplification, although sometimes helpful for clinical decision-making and interpretation of test results, implies that there is a myocardial perfusion threshold at which the benefit of a treatment suddenly appears or disappears. Instead, a more comprehensive evaluation using both PET-derived MBF and FFR will likely allow tailoring of medical therapy and interventions based on their individual disease phenotype, giving an impetus to personalized medicine. This will presumably improve patient satisfaction, temper expectations of invasive procedures, and improve adherence to medical therapy. However, there have not yet been any prospective studies evaluating PET-guided clinical decision-making based on stratification of myocardial perfusion, such as using the concept of coronary flow capacity, ischemic burden, or relative flow ratio.
Limitations
Several limitations need to be acknowledged. First, the Youden index was used to calculate optimal cutoffs. Although the Youden index seeks the optimal balance between sensitivity and specificity, diagnostic accuracy calculations derived from these cutoffs may not consistently translate to the highest attainable diagnostic accuracy given that accuracy is affected by disease prevalence, which differs between specific subpopulations. Second, the present study included a relatively small number of patients aged more than 70 y, which limits the statistical power for accuracy assessment in this subpopulation. Third, we used [15O]H2O as the PET tracer. Consequently, the generalizability of our findings to other PET tracers, such as [13N]NH3, 82Rb, or 18F-flurpiridaz, should be considered with caution. This is the case especially in patients with scarring, as [15O]H2O measures perfusion predominantly in the viable tissue fraction, whereas other PET tracers measure average perfusion in the myocardium. Fourth, the patient groups with reduced global left ventricular ejection fraction or heart failure were mostly excluded. In these patients, the flow capacity is typically reduced because of global myocardial damage, and the same cutoffs may not be valid. Finally, attention was paid to matching visual perfusion defects with their corresponding subtended coronary artery by accounting for coronary dominance. Nevertheless, when a visual defect exceeded more than one coronary territory, following the American Heart Association 17-segment model, mismatches between coronary arteries and their respective vascular regions might have occurred.
CONCLUSION
Optimal cutoffs of quantitative [15O]H2O PET–derived hMBF and CFR to detect FFR-defined significant CAD were similar between patients with prior CAD (prior MI and/or PCI) and patients without prior CAD. Moreover, optimal cutoffs for hMBF were higher in females than males, whereas optimal cutoffs for both hMBF and CFR were lower in patients 70 y or older than in their younger counterparts. Nevertheless, the application of optimized cutoffs within these specific subpopulations did not enhance diagnostic performance. Our findings advocate the use of previously established general cutoffs for detection of FFR-defined significant CAD in the interpretation of myocardial perfusion parameters.
DISCLOSURE
No potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: What is the influence of prior CAD, sex, and age on optimal cutoffs of myocardial perfusion parameters, and does cutoff optimization enhance the diagnostic performance of quantitative [15O]H2O PET for detecting hemodynamically significant CAD?
PERTINENT FINDINGS: The presence of prior CAD did not influence optimal cutoffs of quantitative [15O]H2O PET for detecting FFR-defined hemodynamically significant CAD. For females, however, optimal myocardial perfusion cutoffs were higher than in males. Patients aged more than 70 y had a lower threshold of perfusion parameters than did their younger counterparts. These differences in cutoffs in respective subpopulations pave the way for personalized medicine. Nonetheless, optimizing cutoffs did not exert a positive influence on the diagnostic performance of quantitative [15O]H2O PET for detecting FFR-defined hemodynamically significant disease.
IMPLICATIONS FOR PATIENT CARE: Clinicians can confidently interpret the results of myocardial perfusion imaging based on general nonspecific cutoffs.
Footnotes
Published online May 9, 2024.
- © 2024 by the Society of Nuclear Medicine and Molecular Imaging.
REFERENCES
- Received for publication January 2, 2024.
- Accepted for publication April 8, 2024.