Visual Abstract
Abstract
Radiosynoviorthesis is approved in several European countries and the United States to treat refractory synovitis in many inflammatory joint diseases, such as rheumatoid arthritis, spondyloarthropathies, and other arthritic joint diseases. No radiopharmaceuticals for radiosynoviorthesis are currently approved in Canada. The aim of this Health Canada–approved trial was to demonstrate the safety and efficacy of radiosynoviorthesis. Methods: Between July 2012 and November 2017, we conducted a multicenter, prospective, interventional Canadian trial. Patients (n = 360) with synovitis refractory to standard treatments after failing 2 intraarticular glucocorticoid injections were included. They were followed up at 3, 6, and 12 mo. Outcome measures included adverse events (AEs) and clinical signs of synovitis (pain, swelling, and joint effusion) measured with the Health Assessment Questionnaire Disability Index, the Disease Activity Score, and the Visual Analog Scale. Results: In total, 392 joints were treated, including those reinjected after 6 mo (n = 34). Of these, 83.4% (327/392) were injected with [90Y]Y-citrate for the knees and 9.9% (39/392) with [186Re]Re-sulfide for medium-sized joints. Of the joints treated, 82.7% (324/392) were knees. Fifty-five AEs, most of them of mild grade, occurred and resolved without sequelae and were not life-threatening. The incidence of radiosynoviorthesis-related AEs was 9.4% (34/360). The proportion of patients showing an improvement in synovitis symptoms after radiosynoviorthesis was significant at 3 mo and was maintained up to 12 mo (P < 0.001). Conclusion: This study confirmed the safety of radiosynoviorthesis in the treatment of patients with synovitis refractory to standard treatments. There is evidence of sustained clinical efficacy at 12 mo, suggesting that radiosynoviorthesis is an effective treatment for improving synovitis symptoms.
Radiosynoviorthesis is an intraarticular radionuclide treatment used since 1952 to treat inflammatory joint diseases (1). Radiosynoviorthesis is approved in many European countries and the United States for the treatment of rheumatoid arthritis (RA), spondyloarthropathies, and other arthritic joint diseases (2). The aim of radiosynoviorthesis is to reduce synovitis symptoms after failure or incomplete response to systemic therapy and intraarticular glucocorticoid injections (1). When standard treatment fails, surgical synovectomy may be recommended to remove the inflamed synovial membrane (3,4). Radiosynoviorthesis is an outpatient alternative that delivers equivalent clinical results, is repeatable, reduces the rate of joint effusion after arthroplasty, and is less expensive than surgical synovectomy (5–8).
Previous studies have demonstrated that radiosynoviorthesis is safe. Between 1976 and 2001, 2,412 patients showed no increase in cancer risk, and there was no dose–response relationship with the number of treatments (9). In Europe, of 900,000 joints treated between 1990 and 2011, only 30 severe complications were reported (10). Moreover, radiosynoviorthesis is effective for refractory synovitis. Most studies report an improvement in symptoms during the first 6 mo after the procedure and good symptom control at 12 mo in 70% of patients (11–13). As previously described (14), [90Y]Y-citrate and [186Re]Re-sulfide were available in Canada through a Special Access Program authorization between 1999 and 2010. In 2011, further scientific evidence of the safety and efficacy of radiosynoviorthesis was needed, and to provide such evidence was the aim of this study.
MATERIALS AND METHODS
Patient Population
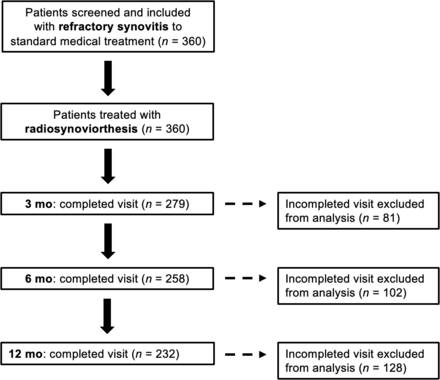
The enrollment flowchart is shown in Figure 1. Between July 2012 and November 2017, all screened patients (n = 360) from 12 hospitals in 5 Canadian provinces were included in this prospective, multicenter, open-label Canadian interventional trial (NCT01615991) approved by Health Canada (approval 9427-C2049-41C) and received radiosynoviorthesis with [90Y]Y-citrate or [186Re]Re-sulfide (IBA-CIS Bio International). There was no minimum or maximum age for inclusion. Patients had to be referred by a specialist physician (rheumatology, internal medicine, or orthopedics) and present clinical signs (pain, swelling, and joint effusion) of active mono- or oligosynovitis. The inflammatory joint diseases included are presented in Table 1. Patients had to have failed medical treatment, defined as the absence of clinical improvement of synovitis symptoms, after 6 mo or 2 intraarticular long-acting glucocorticoid injections. The pain had to limit daily activities or require large doses of analgesics. Patients who had undergone arthroscopic removal of the synovium were eligible. Joint imaging had to show maximally moderate destruction of cartilage or bone (Kellgren–Lawrence class ≤ 3).
Enrollment flowchart.
Patient Characteristics
Exclusion criteria were a joint puncture within the last 2 wk, surgery or arthroscopy within the last 6 wk, radiosynoviorthesis within the last 3 mo, and surgical synovectomy within the last 6 mo in this joint. After a sufficient waiting period, an initially ineligible patient could receive radiosynoviorthesis. Other exclusion criteria included intraarticular fracture, painful prosthesis, massive hemarthrosis, infection, synovial cyst rupture, pregnancy or breastfeeding, bone metastases, allergies to radiopharmaceuticals, and concurrent participation in another clinical trial related to the underlying condition. Data on excluded patients are unknown, as only results from included and treated patients were obtained.
The institutional Research Ethics Board approved this clinical trial (approval CIMS-2011-03), and all subjects gave written informed consent. Each participating site used its own funds to purchase radiopharmaceuticals from the manufacturer, who did not fund the present trial.
Radiosynoviorthesis Procedure
The skin was sterilized with an antiseptic pad saturated with 2% chlorhexidine gluconate and 70% isopropyl alcohol. Sterile drapes and gloves were used. The position of the needle in the synovial space was confirmed by aspiration of synovial fluid from the knee. For other joints, positioning was confirmed by contrast injection under fluoroscopy. [90Y]Y-citrate or [186Re]Re-sulfide was injected for the radiosynoviorthesis. The choice of radiopharmaceutical depended on the size of the joint, and administration was in accordance with the activities and volumes proposed for each radiopharmaceutical in Supplemental Table 1 (supplemental materials are available at http://jnm.snmjournals.org). The [90Y]Y-citrate was used for the knees, and the [186Re]Re-sulfide was used for the hips, shoulders, elbows, wrists, and ankles. On request and under certain circumstances, medium-sized joints could be injected with [90Y]Y-citrate after approval by the steering committee, with the exception of the wrist. Several radiosynoviortheses in different joints during the same visit were possible, but the maximum total activity authorized per visit was 555 MBq with [90Y]Y-citrate and 370 MBq with [186Re]Re-sulfide. The needle was rinsed with methylprednisolone acetate, 40 mg/mL. The volume injected per joint is available in Supplemental Table 2. During needle removal, soft-tissue massage and pressure were applied to the puncture site. The joint had to be immobilized for 48 h. For knees, bed rest without splinting was recommended. If immobilization was not possible, a splint was used to limit movement, as for other joints. Joint scintigraphy was performed within 5 d of the procedure. Patients were followed up at 3, 6, and 12 mo.
Safety
All adverse events (AEs) after radiosynoviorthesis, regardless of grade or relationship to radiosynoviorthesis, were systematically reported by the physician. AEs were graded as mild (no interference with the patient’s usual activities), moderate (disruption of the patient’s usual activities without the need for medical intervention), or severe (disruption of the patient’s usual activities and requiring medical intervention). Moreover, AEs could be definitely related to the treatment, could be probably related (defined as a plausible chronology but potentially attributable to another underlying medical problem), or could be unrelated (defined as a cause-and-effect relationship that is not biologically plausible because of chronology). The safety profile was assessed on the basis of the incidence of AEs described per patient, category, and grades. A joint reaction requiring intraarticular glucocorticoid injection within 14 d of radiosynoviorthesis was considered an AE.
Efficacy
Without clinical improvement in synovitis symptoms (pain, swelling, and joint effusion), treatment failure was defined as the need for an intraarticular glucocorticoid injection more than 14 d after radiosynoviorthesis. A partial response was defined as the need for a second radiosynoviorthesis within the same joint after 6 mo, and steering committee approval was required.
At baseline and at 3, 6, and 12 mo, the patient’s functional disability using the Health Assessment Questionnaire Disability Index (HAQ-DI) (15), the disease activity using the Disease Activity Score (DAS28) (16), and the patient’s global assessment of disease activity using the Visual Analog Scale (VAS 100 mm) (17) were assessed. To be significant, HAQ-DI was to be reduced by 0.25, DAS28 by 1.2, and VAS 100 mm by 20 mm. The proportion of patients with improvement in pain, swelling, and joint effusion at 3, 6, and 12 mo was recorded.
Statistical Analysis
Patients were not excluded from the trial on the basis of missing data; on the contrary, for each variable or multivariate analysis, the maximum number of evaluable patients was used and reported. On the basis of 2009–2010 data available for radiosynoviorthesis performed in Canada under the Special Access Program, the initial target sample size was 1,500 joints. The trial was interrupted before reaching the predetermined number of joints because of a lack of financial resources and time to maintain the steering committee.
Descriptive statistics included mean, median, range, SD, or proportion with a 95% CI, as appropriate. For the incidence of patients with radiosynoviorthesis-related AEs, a 95% Clopper–Pearson CI is provided. The generalized estimating equation model was used to consider the repeated measurements for the main outcomes. If a specific time point was relevant, the McNemar test was used to compare evolution over time. All tests were 2-sided, with a significant probability threshold of 5% (P < 0.05). Analyses were performed using SPSS version 26.0 (IBM).
RESULTS
Patient Characteristics
Table 1 shows the patient characteristics at baseline, when the class of radiologic joint destruction was measured using the Kellgren and Lawrence system (18). A total of 360 patients and 392 joints, including those reinjected after 6 mo (n = 34), underwent radiosynoviorthesis. Before radiosynoviorthesis, 93.6% (337/360) of patients had failed 2 intraarticular glucocorticoid injections. The mean number ± SD of intraarticular glucocorticoid injections received in the past 12 mo was 2.5 ± 0.2. Radiosynoviorthesis was preferable to surgery in 87.2% (314/360) of patients.
Safety
The median patient follow-up time was 12 mo (range, 0–12 mo). The total number of AEs was 55 (Supplemental Table 3). The incidence of patients with radiosynoviorthesis-related AEs was 9.4% (34/360; 95% CI, 6.6%–13.0%). The categories and grades according to the frequency of AEs are presented in Table 2; 14.5% (8/55) of the AEs were severe, 51% (28/55) were mild, 23.6% (13/55) were moderate, and 10.9% (6/55) were of unknown severity. Only 23.6% (13/55) of AEs were definitely related to radiosynoviorthesis, 38.2% (21/55) were possibly related, 27.3% (15/55) were unrelated, and 10.9% (6/55) were of unknown relation. Among the 8 severe AEs, there were 3 cases of radiosynoviorthesis-related radiation synovitis, only one of which resolved with minor sequelae. The other two resolved without sequelae. There was one case of radiation skin necrosis that was severe, occurring at 7 mo and possibly related to radiosynoviorthesis, and a second case that was mild. A single case each of knee synovitis, septic arthritis, lymphadenopathy, and medication error was severe but not related to radiosynoviorthesis as reported by the physician. Systemic reactions (40.0%, 22/55) were attributable to intraarticular glucocorticoid injection. Additional subgroup analyses between spondyloarthropathies, RA, osteoarthritis, pigmented villonodular synovitis, hemophilic arthropathy, and juvenile idiopathic arthritis showed no significant differences in the incidence of AEs or treatment failure reported below.
Categories and Grades of AEs
Efficacy
A total of 16.8% (66/392; 95% CI, 13.3%–20.9%) of joints received an intraarticular glucocorticoid injection after 14 d, corresponding to therapeutic failure. Reinjection after 6 mo was needed in 8.7% of joints (34/392; 95% CI, 6.1%–11.9%), corresponding to a partial response. Among them, 17.6% (6/34) had RA, 14.7% (5/34) had osteoarthritis, and 67.6% (23/34) had diseases of other categories.
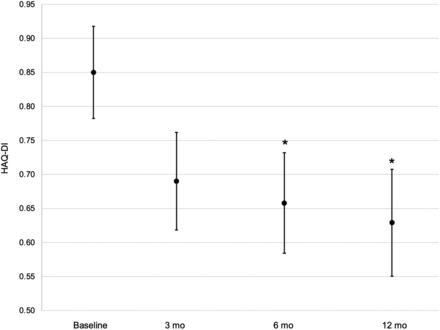
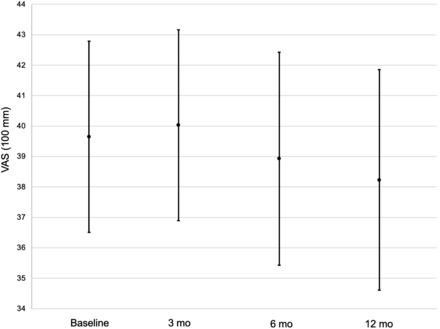
Figure 2 shows the proportion of patients who, after 3 mo, showed a significant improvement (P < 0.001) in synovitis symptoms (pain: 60.4% [95% CI, 53.7%–66.9%] at 3 mo, 57.9% [95% CI, 50.8%–64.6%] at 6 mo, and 98.3% [95% CI, 95.4%–99.5%] at 12 mo; swelling: 63.2% [95% CI, 56.7%–69.6%] at 3 mo, 83.7% [95% CI, 77.9%–88.3%] at 6 mo, and 96.3% [95% CI, 92.5%–98.5%] at 12 mo; and joint effusion: 83.8% [95% CI, 77.8%–88.3%] at 3 mo, 92.6% [95% CI, 87.9%–95.7%] at 6 mo, and 92.4% [95% CI, 87.2%–95.6%] at 12 mo). Figure 3 shows a nonsignificant reduction in the patient’s functional disability (mean HAQ-DI ± SD: 0.85 ± 0.07 at baseline, 0.69 ± 0.07 at 3 mo, 0.66 ± 0.07 at 6 mo, and 0.63 ± 0.08 at 12 mo; P = 0.3). On the other hand, the probability of HAQ-DI improvement decreased statistically significantly at 6 and 12 mo (generalized estimating equation odds ratio: 0.55 [95% CI, 0.35–0.86] at 6 mo [P < 0.01] and 0.42 [95% CI, 0.26–0.66] at 12 mo [P < 0.01]). DAS28 showed a nonsignificant reduction in disease activity (mean DAS28 ± SD: 2.9 ± 0.1 at baseline, 2.6 ± 0.1 at 3 mo, 2.7 ± 0.1 at 6 mo, and 2.6 ± 0.1 at 12 mo [P = 0.1]; generalized estimating equation odds ratio: 0.81 [95% CI, 0.42–1.54] at 6 mo [P = 0.52] and 0.95 [95% CI, 0.52–1.73] at 12 mo [P = 0.86]) (Fig. 4). Figure 5 shows the stability of global assessment of disease activity based on VAS 100 mm (mean VAS ± SD: 39.7 ± 3.1 at baseline, 40.0 ± 3.1 at 3 mo, 38.9 ± 3.5 at 6 mo, and 38.2 ± 3.6 at 12 mo [P = 0.5]; generalized estimating equation odds ratio: 1.14 [95% CI, 0.54–2.39] at 6 mo [P = 0.74] and 1.20 [95% CI, 0.68–2.13] at 12 mo [P = 0.53]). Additional subgroup analyses to compare the efficacy of radiosynoviorthesis between spondyloarthropathies, RA, osteoarthritis, pigmented villonodular synovitis, hemophilic arthropathy, and juvenile idiopathic arthritis showed no significant differences. No progression of the joint destruction initially present was observed, this joint destruction being observed in 32.7% (128/392; 95% CI, 28.0%–37.5%) of joints between baseline and 12 mo. Improvement in joint function at 12 mo was noted by physicians in 74.6% (91/122; 95% CI, 65.9%–82.0%) of patients.
Percentage of patients with reduction in pain, swelling, and joint effusion at 3, 6, and 12 mo after radiosynoviorthesis. Upper brackets indicate 95% CI. Number of evaluable patients is 225 at 3 mo, 211 at 6 mo, and 188 at 12 mo for pain; 229 at 3 mo, 213 at 6 mo, and 189 at 12 mo for swelling; and 207 at 3 mo, 200 at 6 mo, and 178 at 12 mo for joint effusion. *Significant improvement in synovitis symptoms compared with baseline (P < 0.001).
Improvement in patient’s functional disability based on HAQ-DI at baseline and during follow-up (3, 6, and 12 mo). Data are mean; brackets represent SD. Nonsignificant reduction in patient’s functional disability was observed at follow-up compared with baseline (P = 0.3). Smallest clinically significant difference in HAQ-DI is 0.25. Number of evaluable patients is 360 at baseline, 325 at 3 mo, 303 at 6 mo, and 251 at 12 mo. *Probability of HAQ-DI improvement decreases statistically significantly at 6 and 12 mo (P < 0.01).
Decrease in disease activity based on DAS28 at baseline and during follow-up (3, 6, and 12 mo). Data are mean; brackets represent SD. No significant reduction in disease activity was observed at follow-up compared with baseline (P = 0.1). Smallest clinically significant difference in DAS28 is 1.2. Number of evaluable patients is 299 at baseline, 265 at 3 mo, 240 at 6 mo, and 200 at 12 mo.
Stability of patient’s global assessment of disease activity based on VAS at baseline and during follow-up (3, 6, and 12 mo). Data are mean; brackets represent SD. No significant change in patient’s global assessment of disease activity was observed at follow-up compared with baseline (P = 0.5). Smallest clinically significant difference in VAS 100 mm is reduction of 20 mm. Number of evaluable patients is 286 at baseline, 255 at 3 mo, 238 at 6 mo, and 202 at 12 mo.
DISCUSSION
In this study, mild and severe AEs occurred in 5.8% (21/360; 95% CI, 3.6%–8.8%) of patients. Most AEs were mild and resolved spontaneously. Other studies have also shown that radiosynoviorthesis using [90Y]Y-citrate and [186Re]Re-sulfide is safe and well tolerated (11,19–21), with no evidence of radiation-induced cancers at the long-term follow-up (9,22,23). In this trial, one of the severe complications requiring surgical and multidisciplinary management was radiation skin necrosis at the wrist injection site at 7 mo (14). The second case of radiation skin necrosis was mild, and both cutaneous complications resolved without sequelae. This serious complication occurred in only 0.6% of cases in our trial. In the literature, radiation skin necrosis is a complication occurring in 1.2% of patients (24,25). The choice of radioisotope is decisive in preventing this complication. Indeed, the extent of energy deposition in soft tissue by the chosen radioisotope is crucial, which explains why wrists with a thinner synovium benefit more from medium-energy radioisotopes, such as [186Re]Re-sulfide (mean range, 1.2 mm), than from [90Y]Y-citrate (mean range, 3.6 mm) (2). For this reason, even on request, treatment of a wrist with [90Y]Y-citrate was not permitted in this trial.
Only one case of severe septic arthritis of the knee occurred, representing an incidence of 0.3%. This is in line with the 0.1% incidence of septic arthritis reported in the literature (26). Septic arthritis after radiosynoviorthesis is attributable to the procedure itself, not the radiopharmaceuticals, and is most often secondary to skin bacteria (2,27). A few studies have shown that certain risk factors, including diabetes, a prosthesis, and glucocorticoid coadministration during radiosynoviorthesis, were more likely to increase the risk of infection (27,28). Here, because of the lengthy interval between the procedure and the AE (12 mo), septic arthritis of the knee is explained by the patient’s multiple underlying comorbidities rather than the radiosynoviorthesis.
In our study, the significant and maintained improvement in synovitis symptoms supports the efficacy of radiosynoviorthesis for the treatment of refractory synovitis. A systematic review with metaanalyses also demonstrated the efficacy of radiosynoviorthesis (29). For [186Re]Re-sulfide radiosynoviorthesis, the success rate was 69%–100% at 6 mo and 54%–100% at more than 12 mo, compared with [90Y]Y-citrate, for which the success rate was 24%–100% at 6 mo and 29%–94% at 12 mo. In our study, clinical improvement in joint function was seen in 73.3% of patients at 6 mo and in 74.6% at 12 mo. Our results are in line with a metaanalysis of 2,190 joints for which the overall response rate was 72.5% (30). There was also a significant early reduction in synovitis symptoms at 3 mo that was sustained at 12 mo. Some studies have also shown an early clinical response as early as 6 wk and a significant sustained response 10 y after radiosynoviorthesis (11,12,19,26). In addition, there was no progression of the joint destruction initially present. A 9-y study of 153 joints showed no structural progression in 80% of joints treated (31). Only 16.8% of treated joints required one or more intraarticular glucocorticoid injections after more than 14 d, confirming the therapeutic efficacy of radiosynoviorthesis. RA and osteoarthritis were the main underlying inflammatory conditions in the reinjected joints, but there was no significant difference between these groups for efficacy. This can be explained by the small cohort in our study and the presence of missing data. A study including 38 joints mainly affected by RA showed that a second attempt had a certain advantage (11). Other studies have reported better results for RA than for osteoarthritis (26,30). Finally, all joints in our study received a therapeutic dose of methylprednisolone acetate, 40 mg/mL, during radiosynoviorthesis for needle flushing. A 3-arm, double-blind study comparing [90Y]Y-citrate with [90Y]Y-citrate and glucocorticoids, and with glucocorticoids alone, demonstrated superior efficacy of [90Y]Y-citrate versus glucocorticoids at 12 mo (32). This procedure probably did not influence the efficacy of radiosynoviorthesis.
Our study had a few limitations. Several variables, including blood tests, bone scans, and medical questionnaires, were not collected at the main site, resulting in several missing data. For example, response assessment by bone scintigraphy at 6 and 12 mo was not performed for this reason. Only 38.8% of bone scans were collected at the initial assessment, compared with 26.6% at 12 mo. However, one study involving 89 joints showed an improvement of over 68% in synovitis symptoms when followed up with 2-phase bone scans (31). Another limitation of the study is the absence of a comparator arm. However, our study demonstrated efficacy of 74.6% at 12 mo; this efficacy is superimposed on the success rates found in the literature, which range from 60% to 80% for radiosynoviorthesis and surgical synovectomy (6,26). As all patients received intraarticular glucocorticoid injections during radiosynoviorthesis, there may be a favorable or synergistic response to this combination therapy. Other studies have used the same approach and obtained results similar to ours (25,26).
CONCLUSION
The results of this Canadian cohort confirm the safety of radiosynoviorthesis with [90Y]Y-citrate and [186Re]Re-sulfide while showing this treatment to be efficacious for patients with refractory synovitis. The results also demonstrate that radiosynoviorthesis is a safe alternative to surgical synovectomy, for example—particularly in candidates with multiple comorbidities for whom surgery is undesirable—and significantly reduces synovitis symptoms in a majority of patients.
DISCLOSURE
No potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: Is radiosynoviorthesis safe and effective?
PERTINENT FINDINGS: In this prospective, multicenter, open-label Canadian interventional trial of 360 patients and 392 joints treated with [90Y]Y-citrate or [186Re]Re-sulfide radiosynoviorthesis, the incidence of patients with radiosynoviorthesis-related AEs was 9.4%, the majority being mild and without sequelae. A significant and prolonged improvement in synovitis symptoms was also observed at 12 mo.
IMPLICATIONS FOR PATIENT CARE: Health Canada’s official approval of radiopharmaceuticals for radiosynoviorthesis, a safe and effective treatment, would improve the quality of life of patients with synovitis refractory to standard treatments.
ACKNOWLEDGMENTS
Special thanks are due to the Canadian coinvestigators of this research for their active participation in data collection, synoviorthesis, and participant follow-up: Dr. Joël Desroches (CHRTR, Quebec), Dr. Marcel Dumont (CHUQ–CHUL, Quebec), Dr. Michel Picard (CHUM, Quebec), Dr. J. Carter Thorne (Southlake Regional Health Centre, Ontario), Dr. Andrew Ross (QEII Hospital, Nova Scotia), Dr. Daniel Dionne (CSSS de Rimouski Neigette, Quebec), Dr. Jean-Mathieu Beauregard (CHUQ–Hôtel-Dieu and Hôpital Maisonneuve, Quebec), Dr. Grégoire Blais (CH Granby, Quebec), Dr. Raymond Taillefer (CH Ste-Croix, Quebec), Dr. Lucie Carrier (Hôpital Pierre-Boucher, Quebec), Dr. François Raymond (CSSS de Gatineau, Quebec), Dr. Eugene Leung (Ottawa Hospital, Ontario), Dr. Rachel Shupack (St. Michael’s Hospital, Ontario), Dr. Bohdan Bybel (Winnipeg Health Science Centre, Manitoba), Dr. Elaine Joughin (Alberta Children’s Hospital, Alberta), Dr. Philip Cohen (Lions Gate Hospital, British Columbia), and Dr. Jonathan Romsa (Victoria Hospital LHSC, British Columbia). We thank the biostatistics department of the Research Center of CHUS (CRCHUS) for its support in the statistical analysis.
Footnotes
Published online May 16, 2024.
- © 2024 by the Society of Nuclear Medicine and Molecular Imaging.
REFERENCES
- Received for publication December 18, 2023.
- Accepted for publication April 22, 2024.