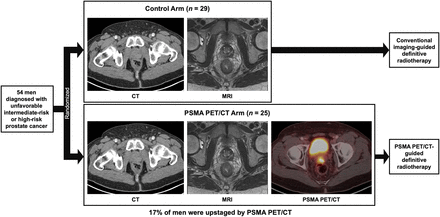
Visual Abstract
Abstract
This multicenter randomized phase III trial (NCT04457245) evaluated the effect of performing prostate-specific membrane antigen (PSMA) PET/CT before definitive radiotherapy. Methods: Men with unfavorable intermediate- or high-risk prostate cancer were randomized 1.08:1 between receiving and not receiving a PSMA PET/CT scan before definitive radiotherapy. All other imaging modalities were allowed in the control arm. The primary endpoint was 5-y progression-free survival. Results: Fifty-four men were randomized between November 2020 and December 2021 (PSMA PET/CT, n = 25; control, n = 29). The trial closed early after approval and insurance coverage of PSMA PET/CT. In the PSMA PET/CT arm, 14 patients had localized disease (miT2b-cN0M0), 6 had locally advanced disease (miT3a-bN0M0), 3 had regional metastasis (miN1M0), and 1 had distant metastasis (miM1b). Four patients were upstaged. Conclusion: PSMA PET/CT upstaged 17% of patients, which allowed for more accurate radiotherapy planning. Unfortunately, this trial closed early before completion of target enrollment (54/316, 17%) and was underpowered to assess the effect of PSMA PET/CT on progression-free survival.
Prostate-specific membrane antigen (PSMA) is highly expressed in prostate cancer cells, making it an excellent target for PET radiotracers used to detect prostate cancer. PSMA PET/CT offers superior diagnostic accuracy for nodal and distant metastasis compared with both conventional imaging (CT, bone scanning, and MRI) (1,2) and non-PSMA radiotracer PET scans (3–5).
Nonrandomized studies that used PSMA PET/CT for staging before definitive radiotherapy (dRT) reported distant metastasis in 6%–9% of patients and findings that led to either radiation dose escalation or pelvic lymph node irradiation in 13%–20% of patients (6–9). However, the effect of PSMA PET/CT staging before dRT on clinical outcomes has not been well studied in a randomized controlled trial. Here, we discuss the results of the PSMA-dRT phase III randomized controlled trial.
MATERIALS AND METHODS
Study Population
The PSMA-dRT trial was a multicenter phase III randomized controlled trial (ClinicalTrials.gov identifier NCT04457245). This was an investigator-initiated trial supported by Progenics Pharmaceuticals Inc., conducted under investigational new drug application 147591. The UCLA institutional review board approved this study (approval 20-000378) and all subjects gave written informed consent. The study protocol (supplemental material, available at http://jnm.snmjournals.org) was previously published (10).
This study was designed to randomize 312 men with unfavorable intermediate- or high-risk prostate cancer 1.08:1 between receiving and not receiving a PSMA PET/CT scan. This randomization was chosen to account for an estimated detection rate of 8% for extrapelvic metastasis on PSMA PET/CT. Patients in the intervention arm who were found to have extrapelvic metastasis were no longer eligible for dRT and were not included in the primary endpoint analysis. In the intervention arm, reports and DICOM images of the PSMA PET/CT were transferred to the treating radiation oncologist before radiotherapy planning. In the control arm, patients were staged per physician discretion (CT, MRI, bone scanning, or PET/CT with a non-PSMA radiotracer).
Radiotherapy Delivery
Radiotherapy was delivered at the treating radiation oncologist’s facility. The treating radiation oncologist decided on the radiation modality (external-beam radiotherapy, low-dose-rate brachytherapy, high-dose-rate brachytherapy, or external-beam radiotherapy with a brachytherapy boost), radiation dose, fractionation (conventionally fractionated, moderately hypofractionated, or stereotactic body radiotherapy), inclusion of elective pelvic lymph nodes, and inclusion of androgen deprivation therapy.
Patient Follow-up
Patients had follow-up visits with the treating radiation oncologist every 3–4 mo for the first year and every 6 mo thereafter. Patients underwent prostate-specific antigen testing around the time of each follow-up visit. Imaging follow-up was ordered per physician discretion if disease progression was suspected on the basis of a rising prostate-specific antigen level.
Study Endpoints
The primary endpoint was progression-free survival (PFS) at 5 y. Progression was defined as whichever of the following occurred first: prostate-specific antigen level more than 2 ng/mL above the postradiotherapy nadir, recurrence on imaging or biopsy, initiation of salvage therapy, or death. Progression was calculated starting from the time of randomization.
Statistical Analysis
Differences in patient and treatment characteristics between the 2 cohorts were compared using the Pearson χ2 test for categoric variables and 2-tailed t tests for continuous variables. PFS was calculated using Kaplan–Meier survival analysis. Comparisons were made using log rank testing. All statistical analyses were performed using SPSS Statistics, version 28 (IBM Corp.).
RESULTS
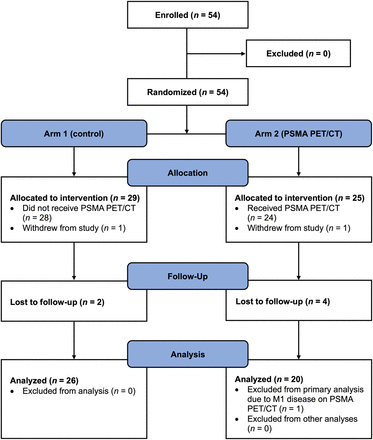
Between November 2020 and December 2021, 54 patients were randomized (PSMA PET/CT, n = 25; control, n = 29). Two patients withdrew after randomization and were excluded (Fig. 1). Table 1 describes patient and treatment characteristics. There were no significant differences between the 2 groups.
CONSORT (Consolidated Standards of Reporting Trials) flow diagram.
Patient and Treatment Characteristics
Among patients staged using PSMA PET/CT in the intervention arm (n = 24), 14 had localized disease (miT2b-cN0M0), 6 had locally advanced disease (miT3a-bN0M0), 3 had regional metastasis (miN1M0), and 1 had regional and distant metastasis (miN1M1b). Four were upstaged relative to baseline: 1 with locally advanced disease, 2 with regional metastasis, and 1 with distant metastasis.
All 3 patients with miN1M0 disease received pelvic lymph node irradiation. The patient with miN1M1b disease received upfront androgen deprivation therapy with abiraterone acetate and prednisone followed by consolidative radiotherapy to the prostate and pelvic lymph nodes.
After U.S. Food and Drug Administration approval of PSMA PET/CT radiotracers in 2021, patients gained access to PSMA PET/CT as a standard, medically reimbursed procedure. Consequently, the trial closed prematurely in February 2022 after the recruitment rate significantly decreased.
At the time of this analysis (August 2023), median follow-up was 21 mo (interquartile range, 17.6–26.3 mo). There were no cases of biochemical recurrence, disease recurrence, or prostate cancer–specific death in either arm. There were 2 nonprostate cancer deaths in the PSMA PET/CT arm. Two-year PFS was 93.8% for the PSMA PET/CT arm and 100% for the control arm. There were no significant differences between the 2 groups (Fig. 2, P = 0.13).
PFS: control and PSMA PET/CT cohorts were compared using log rank testing. Ticks represent censored cases. Patients with extrapelvic metastasis on PSMA PET/CT who were not eligible for dRT were excluded (1 patient).
DISCUSSION
In this study of men with unfavorable intermediate- or high-risk prostate cancer, PSMA PET/CT upstaged 1 in 6 patients relative to baseline staging. This information guided target volume delineation, radiation dose, and hormone therapy intensification. This suggests that the adoption of PSMA PET/CT for primary staging in this patient population impacts management choices.
Unfortunately, this study was terminated prematurely after Food and Drug Administration approval of PSMA PET/CT radiotracers. Before Food and Drug Administration approval, there was significant interest in this trial among patients and enrolling physicians because of the otherwise significant hurdles to obtaining PSMA PET/CT. After Food and Drug Administration approval, most medical insurance companies in the United States covered PSMA PET/CT scans for primary staging of men with high-risk or unfavorable intermediate-risk prostate cancer, significantly reducing enrollment and the feasibility of the control arm.
As a result, this trial was closed after randomizing only 54 men, thus significantly reducing the statistical power of this study to detect a PFS difference. A second limitation is the low frequency of biochemical recurrence and disease progression in the first 2 y after dRT. Even in cohorts that include men with high-risk prostate cancer, reported 2-y rates are less than 5% (11). Thus, the only observed PFS events were 2 nonprostate cancer deaths in the PSMA PET/CT arm.
When examining upstaging rates of PSMA PET/CT compared with other imaging modalities, we found consistency with previously reported, prospective trials. The proPSMA study reported that PSMA PET/CT led to more frequent management changes than did conventional imaging (28% vs. 15%, P = 0.008) (1). A prospective, multicenter study in Australia by Roach et al. found that PSMA PET/CT changed management in 21% of cases (9). These are both similar to the 16.7% reported in our study.
Across multiple prospective studies, PSMA PET/CT has been shown to have superior accuracy and sensitivity for staging patients compared with conventional imaging. This information can guide radiation volume delineation, radiation dose escalation, and hormone therapy intensification. The rapid adoption and availability of PSMA PET/CT staging has already changed prostate cancer treatment across the world. As a result of its success, the window to conduct prospective randomized controlled trials and show long-term clinical outcome benefits is closing. This raises questions about the expectations of the medical community to first demonstrate improved oncologic outcomes before widespread adoption. Including PSMA PET/CT staging in the design of future clinical trials is now warranted, and strong consideration must be given to how and whether PSMA PET/CT-based endpoints should be included as primary or secondary endpoints in clinical trials.
CONCLUSION
PSMA PET/CT upstaged 17% of patients, which allowed for more accurate radiotherapy planning. Unfortunately, this trial was underpowered to assess the effect of PSMA PET/CT on progression-free survival.
DISCLOSURE
This is an investigator-initiated trial with institutional funding (UCLA Ahmanson Translational Theranostics Division). This study is supported by Progenics Pharmaceuticals Inc., which supplies the study drug (18F-DCFPyL) and financial support for the PET/CT technical costs associated with the study under the PyL Research Access Program (Progenics Pharmaceuticals, Inc.). John Nikitas received funding from the Christiaan W. Schiepers Theranostics Fellowship award. Jeremie Calais reported consulting fees from AAA, Astellas, Blue Earth Diagnostics, Curium Pharma, DS Pharma, EXINI, GE Healthcare, Isoray, IBA RadioPharma, Janssen, Lightpoint Medical, Lantheus, Monrol, Novartis, Progenics, POINT Biopharma, Radiomedix, Sanofi, and Telix Pharmaceuticals. Amar Kishan reported personal fees from ViewRay, Varian Medical Systems, and Janssen Pharmaceuticals and research funding from ViewRay. Boris Hadaschik reported serving on advisory boards for Janssen, Bayer, ABX, Lightpoint, Amgen, MSD, Pfizer, and Novartis; serving as an invited speaker for Accord, Astellas, and Janssen R&D; receiving honoraria from Uromed; receiving research support from AAA/Novartis, Bristol Myers Squibb, and the German Research Foundation; and having a leadership role with DKG, AUO, and DGU. Nicholas Nickols reported research grants from Janssen, Lantheus, and Bayer and personal fees from OncoLinea. Michael Steinberg reported receiving consulting fees from ViewRay. No other potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: What is the effect of performing PSMA PET/CT before dRT for prostate cancer?
PERTINENT FINDINGS: One in 6 patients was upstaged by PSMA PET/CT. This study closed early before completion of target enrollment (54/316, 17%) and was underpowered to assess the effect on PFS.
IMPLICATIONS FOR PATIENT CARE: Information from PSMA PET/CT can guide radiation volume delineation, radiation dose escalation, and hormone therapy intensification.
Footnotes
Published online Apr. 25, 2024.
- © 2024 by the Society of Nuclear Medicine and Molecular Imaging.
REFERENCES
- Received for publication November 3, 2023.
- Accepted for publication March 28, 2024.