Visual Abstract
Abstract
The aim of this work is to evaluate our clinical real-world data obtained with 225Ac-PSMA-617 (AcPSMA), which were acquired under compassionate care regulations in patients with advanced-stage prostate cancer. The objective parameters that could be derived from this evaluation are compared with previous literature about AcPSMA and 177Lu-PSMA-617 (LuPSMA). Methods: The medical files of all patients who had received AcPSMA on an individual patient basis at the Heidelberg University Hospital since January 2014 were analyzed retrospectively. Previously published patients were excluded. The remaining patients were tailored into 2 subgroups with different treatment strategies: group 1 received AcPSMA as a deescalated monotherapy, and group 2 received LuPSMA plus AcPSMA as a cocktail regimen. Baseline characteristics, serum prostate-specific antigen (PSA) response, and overall survival were compared with the most appropriate historical controls. Results: Of 287 patients treated, 54 were excluded because of previous publication and 233 were evaluated, 104 of whom received AcPSMA monotherapy (median, 6 MBq). In this group, 55 patients (53%) presented with a best PSA response of at least 50%. The other 129 patients received a cocktail therapy of AcPSMA (median, 4 MBq) plus LuPSMA (4 GBq). In this group, a best PSA response of at least 50% was observed in 74 patients (57%). The median overall survival in the monogroup was 9 mo and in the cocktail group was 15 mo. If adjusted for prognostic baseline characteristics, the efficacy of both regimens was not significantly different. Conclusion: Deescalated treatment activities of AcPSMA or AcPSMA and LuPSMA cocktail regimens present better tolerability with regard to xerostomia than previous regimens of at least 100 kBq/kg while retaining high antitumor activity in poor-prognosis prostate cancer patients.
Prostate-specific membrane antigen (PSMA)–targeted α-therapy (TAT) for advanced-stage metastatic castration-resistant prostate cancer patients was introduced in 2014 (1). After a preliminary dosimetry estimate and brief empiric dose escalation (2), treatment activity of 100 kBq of 225Ac-PSMA-617 (AcPSMA) per kilogram of body weight (kgBW) was proposed and applied to the next 40 patients (3). Using this protocol, promising efficacy with regard to duration of tumor control has been observed. However, salivary gland toxicity increased with cumulative treatment cycles and 10% of responding patients discontinued PSMA-TAT because of dose-limiting xerostomia.
In the following years and confirmative to one another, some research groups reported a relevant tumor sink effect for radiolabeled PSMA ligands in high-tumor-burden patients (4–6). Because our initial dose escalation was based on such advanced patients, it is likely that we overestimated the maximum tolerable dose with respect to low to intermediate tumor mass patients, including the ones who started PSMA-TAT with high tumor volume but achieved partial remission after the first treatment cycles. With growing acceptance of PSMA-targeted radiopharmaceutical therapy in general, an increasing number of patients with less advanced tumor spread were scheduled to our department to receive PSMA-TAT. Thus, the responsible nuclear medicine physicians often modified the regimen by reducing the treatment activity.
Some years ago, a pilot study introduced the concept of 177Lu-PSMA-617 (LuPSMA) and AcPSMA combination therapy (7). One course of AcPSMA (median, 5.3 MBq; range, 1.5–7.9) combined with LuPSMA (median, 6.9 GBq; range, 5.0–11.6) was administered to 20 metastatic castration-resistant prostate cancer patients and followed by LuPSMA maintenance monotherapy. In a different publication from the same group, 2 cycles of LuPSMA (mean, 6.7 ± 1.8 GBq) were administered to 15 patients and augmented with 2.7 ± 1.1 MBq of AcPSMA in the first (n = 7), second (n = 3), or both (n = 5) cycles (8). In a third publication, 17 patients received a single cycle of 1.8–6.9 MBq of AcPSMA in combination with 3.8–8.2 GBq of LuPSMA (9). The salivary gland toxicity of these combination therapies was found to be favorable compared with 100 kBq of AcPSMA per kgBW and comparable to LuPSMA standard therapy. These preliminary reports have relevant limitations because of the variability in the numbers of performed treatment cycles, the range of administered activity of both LuPSMA and AcPSMA, short follow-up, low overall patient numbers, and major heterogeneity on how treatment was continued after the 1–2 cycles of combination therapy (i.e., some patients received a further combination and others received maintenance with LuPSMA only). Nevertheless, because of the low rate of reported xerostomia at promising antitumor activity, we considered the cocktail approach to be an encouraging strategy to refine the trade-off between tolerability and antitumor activity.
In this retrospective evaluation, we determined the tolerability, the prostate-specific antigen (PSA) response rate, and overall survival (OS) observed with patients receiving a regimen of less than 100 kBq of AcPSMA per kgBW or AcPSMA and LuPSMA cocktail therapy.
MATERIALS AND METHODS
Patients
We retrospectively analyzed all patients who started treatment with AcPSMA in the Department of Nuclear Medicine at Heidelberg University Hospital between January 2014 and July 2021. These patients were considered inappropriate for treatment or had already exhausted the approved treatments at that time. Experimental salvage therapies were offered on an individual patient basis under the conditions of the updated Declaration of Helsinki, paragraph 37 (“Unproven Interventions in Clinical Practice”), and national regulations. Patients were informed about the experimental nature of this therapy and gave written informed consent. Considering their own clinical experience with AcPSMA, general experience with PSMA-targeted radiopharmaceutical therapy, and patients’ different clinical situations, physicians offered patients a dosing regimen that followed the best available knowledge at the time. Treatment with AcPSMA was offered only to patients with a positive PSMA baseline scan that was available under real-world conditions. For 68Ga-PSMA-11, 18F-DCFPyL, and 18F-PSMA-1007, a SUV of more than 10 was defined as PSMA-positive. For 99mTc-PSMA tracers, tumor uptake equal to or higher than that of salivary glands in visual inspection in all measurable tumor lesions (in analogy to the VISION and TheraP clinical trials, only lesions > 10 mm were considered measurable) was defined as PSMA-positive. This retrospective observational study was approved by the research ethics committee of the medical faculty of Heidelberg University (permit S-732/2018).
Patients who were previously published from our department were excluded from this analysis (1–3). Nevertheless, we cannot exclude partial overlap with pooled patient cohorts of patients treated in Germany that have been reported from a Dutch research group (10,11).
Radiopharmaceuticals, Patient Preparation, and Dosing Regimens
The PSMA-617 precursor was obtained from ABX. 225Ac was produced by radiochemical extraction from 229Th at the European Commission’s Joint Research Centre (12). 177Lu (EndolucinBeta) was obtained from ITM. The labeling conditions for AcPSMA and LuPSMA have been described previously (1,13). The treatment activity was determined on the basis of consensus of those authors who already had broad clinical experience with 131I-MIP1095 (14), 90Y-PSMA-617 (15), LuPSMA (16,17), AcPSMA (2), and 225Ac-DOTATOC (18), considering the prognosis and progression velocity of each patient.
These individual treatment decisions can be assigned to 2 general concepts. The first concept, dynamic deescalation, means that the first cycle was performed with a fixed standard treatment activity of 8 MBq of AcPSMA (a simplification of the protocol of 100 kBq of AcPSMA per kgBW, considering an average body weight of 80 kg for male patients) in superscan-pattern patients and 6 MBq in normal biodistribution patients. Depending on PSA and clinical response, the treatment activity was reduced by 2 MBq for the subsequent cycles (i.e., 4–6 MBq for the second cycle and eventually 2–4 MBq for the third cycle).
The second approach was to administer a combination of approximately 4 GBq of LuPSMA together with 4 MBq of AcPSMA, which equals approximately 50% of the previously recommended LuPSMA and AcPSMA treatment doses, respectively.
In both concepts, the intention-to-treat protocol was a 3-cycle therapy, with cycles administered every 2 mo (8–9 wk). Standard androgen deprivation therapy and bone-protecting agents were continued during AcPSMA, but other therapies related to metastatic castration-resistant prostate cancer were discontinued (chemotherapies > 6 wk in advance).
The treatment was administered as a slow (∼30 s), intravenous freehand injection from a lead-shielded syringe. Hydration with 2 L of physiologic electrolyte solution (Sterofundin; B. Braun Melsungen) was administered on the treatment day (starting 30 min before therapy with a flow of 250 mL/h; combination with oral or intravenous diuretics was clinically indicated) and the following day, respectively.
Follow-up and Statistical Data Analysis
Restaging per PSMA PET/CT or PSMA SPECT/CT was recommended 2 mo after the third treatment cycle (or earlier in cases of clinical and biochemical progression) at the location of the initial staging. In long-term follow-up, the urologist or oncologist chose imaging according to medical appropriateness. PSA was measured at week 8 and week 16 after the first treatment cycle and was evaluated with waterfall graphs for the week 8 PSA response or best PSA response obtained at either week 8 or week 16.
Statistical analysis was performed using SPSS version 28 (IBM). Median OS (mOS) was approximated with Kaplan–Meier curves using the data provided by local oncologists or national death record registers. Patients still alive were censored with their last available follow-up date (data freeze, May 2, 2022). The baseline characteristics of the cohorts were compared using (2-sided) Pearson correlation. OS was checked to determine whether it was related to the presence of bone marrow infiltration, and a significant difference was found between the groups (ANOVA). A propensity score matched-pair analysis (paired t test) was done for OS using the factors of age, Eastern Cooperative Oncology Group clinical performance status score, time to therapy, and presence of bone marrow infiltration.
RESULTS
From 287 patients who received AcPSMA between January 2014 and July 2021, 54 were previously published by our group and therefore excluded from this analysis. The medical files of the remaining 233 patients, who originated from several countries all over the globe (Supplemental Fig. 1 [supplemental materials are available at http://jnm.snmjournals.org]), were evaluated retrospectively. The analysis revealed that between 2014 and 2017, 104 patients received deescalated AcPSMA monotherapy and that between 2018 and 2021, 129 patients received a cocktail regimen.
The main characteristics of the patient cohorts receiving AcPSMA monotherapy and the AcPSMA and LuPSMA cocktail are provided in Table 1. The baseline profile of our patients is relatively similar to that of the patients who have been recruited to the VISION clinical trial (19). However, 5%–16% of our patients would not have been candidates for the VISION trial because of low platelet count and 12%–22% would not have been candidates because of low baseline hemoglobin. Our monotherapy and cocktail groups also included 37% and 32% of patients who had previously been treated with LuPSMA. Both of our groups included more patients with visceral metastases than did the VISION trial.
Patient Characteristics for AcPSMA Monotherapy Group and AcPSMA and LuPSMA Cocktail Group
An itemwise comparison of our patients’ baseline characteristics with the historical controls from the VISION trial is provided in Supplemental Table 1. In our patients, some other nonstandard treatments had been applied in advance of AcPSMA; in contrast, VISION patients received these treatments after LuPSMA (Supplemental Table 2). The baseline characteristics were well balanced between the monotherapy and the cocktail groups except for a significantly higher number of patients with bone marrow infiltration in the monotherapy group (P < 0.001).
In total, 536 treatment cycles with cumulative treatment activity of 2,884 MBq of 225Ac were administered to either 104 patients receiving AcPSMA monotherapy (250 cycles, 1,644 MBq of 225Ac, and average of 6.6 MBq/cycle) or 129 patients receiving AcPSMA and LuPSMA cocktail therapy (286 cycles, 1,240 MBq of 225Ac, and average of 4.3 MBq/cycle).
We did not observe acute adverse reactions during application and observed only moderate (numeric rating scale change of 2–4 compared with administration date) flare-up of pain in bone lesions in 37 of 536 treatments (7%) during the first 48-h observational phase after the injection. Mild xerostomia (Common Terminology Criteria for Adverse Events grade 1 or 2; National Cancer Institute) was reported from all patients. However, patients who discontinued PSMA-TAT did so because of insufficient PSA response, clinical nonresponse, or travel restrictions related to the coronavirus disease 2019 pandemic. In contrast to previous treatment regimens, none of the responding patients discontinued because of intolerable xerostomia.
After the first treatment cycle, of the 104 patients who received AcPSMA monotherapy, 46 (44%) achieved a decrease of more than 50% in the PSA level and 27 (26%) achieved a PSA decrease of more than 80% at week 8. Of the 129 patients who received AcPSMA and LuPSMA cocktail therapy, decreases in PSA of more than 50% and 80% were observed in 64 (50%) and 34 (26%), respectively, at week 8.
In the AcPSMA monogroup, the best PSA response, a PSA decline of more than 50%, was found in 55 of 104 patients (53%), and a PSA decline of more than 80% was found in 39 of 104 patients (38%; Fig. 1A). Best PSA responses of more than 50% were found in 74 of 129 patients (57%; Fig. 1B) and of more than 80% were found in 45 of 129 patients (35%) of the cocktail cohort. Consequently, there was no statistically significant difference in PSA response between the 2 groups (χ2 test with Yates correction, P = 0.49, and without Yates correction, P = 0.58). The PSA response correlates well with the imaging response of the PET-positive volume. Positive radiologic responses of lymph nodal and visceral metastases were observed with both treatment schemes (Fig. 2).
Waterfall graphs of best PSA response. (A) Of 104 patients receiving AcPSMA monotherapy, 55 had PSA decline of >50% (green); 16 had stable disease, defined by PSA decrease of <50% up to PSA increase of <20% (blue); 17 had PSA progression (red); and 16 were follow-up losses (purple). (B) Of 129 cocktail group patients, 74 had PSA decline of >50% (green), 36 had stable disease (blue), 17 had PSA progression (red), and 12 were follow-up losses (purple).
(A–D) Patient with meningeal, adrenal, pulmonary, lymph nodal, and osseous metastases. (A) Baseline staging per 18F-PSMA-1007 PET/CT is demonstrated as maximum-intensity projection. (B and C) Two cycles of AcPSMA and LuPSMA cocktail therapy were documented per planar scan of 208-keV γ-line of 177Lu. (D) PET/CT restaging presents partial remission. (E–H) Patient with adrenal, osseous, lymph nodal, and hepatic metastases. (E) Baseline imaging was done as planar 99mTc-PSMA scintigraphy. (F and G) AcPSMA monotherapy was documented per planar emission scans using 26% 440-keV and 12% 218-keV γ-coemissions of 213Bi and 221Fr. (H) Restaging per planar 99mTc-PSMA scintigraphy demonstrates near-total remission.
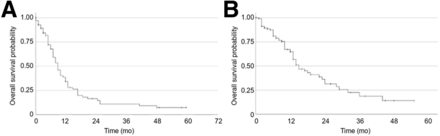
The mOS of the AcPSMA monotherapy patients was 9.0 mo (95% CI, 7.2–10.8 mo), and the mOS of the cocktail therapy patients was 15.0 mo (95% CI, 11.0–19.0 mo). Kaplan–Meier curves are presented in Figure 3.
Kaplan–Meier curves for AcPSMA monotherapy group with mOS of 9 mo (95% CI, 7.18–10.82) (A) and for AcPSMA and LuPSMA cocktail group with mOS of 15 mo (95% CI, 10.93–19.02) (B).
In ANOVA, OS was significantly (P = 0.032) dependent on the presence of bone marrow infiltration. Because the percentage of bone marrow–infiltrated patients was significantly (P < 0.001) higher in the monotherapy group, most likely the poor risk profile of this group is responsible for the shorter mOS observed. If a propensity score matched-pair analysis considering the Eastern Cooperative Oncology Group clinical performance status score, bone marrow infiltration, and time from diagnose to PSMA therapy is applied, the mOS between the 2 groups is not significantly different (P = 0.174).
DISCUSSION
In this work, we retrospectively compare 2 approaches to improve the tolerability of PSMA-TAT to salivary glands without losing too much antitumor activity. One group of patients received monotherapy with AcPSMA that was individually deescalated by considering baseline tumor burden and early PSA response, and another group received combination therapy of AcPSMA plus LuPSMA. PSA response and OS are not significantly different between the groups if propensity score matching for the Eastern Cooperative Oncology Group clinical performance status score and bone marrow infiltration is applied.
With our initially projected regimen of 100 kBq of AcPSMA per kgBW, a PSA response of more than 50% in 24 of 40 intention-to-treat patients (60%) was observed. However, 4 of 40 patients (10%) discontinued therapy because of xerostomia despite positive response (3). This dosing regimen has been used in other centers. Satapathy et al. (20) reported 11 patients (46%) with a PSA response of more than 50%; the follow-up was too short to assess mOS. Yadav et al. (21) reported 28 patients with a median follow-up of up to 22 mo and found that 39% of these patients had a PSA response of more than 50%, with a mOS of 17 mo. Previous chemotherapy in 82% (23/28) and the fraction of patients with previous LuPSMA (15/28) are comparable to our baseline characteristics (i.e., previous chemotherapy in 85% and 68% and previous LuPSMA in 37% and 32% of the AcPSMA monotherapy group and the cocktail group, respectively) (21). The fractions of cocktail group patients with lung, liver, and brain metastases were 10.7%, 10.7%, and 7%, respectively, versus 13%, 22%, and 2%, respectively, in our monotherapy group patients (21). Even higher treatment activities of 100–150 kBq/kg were used by Ballal et al. (22) and translated into a higher rate of treatment-related fatigue. Nevertheless, PSA response and mOS demonstrated no clear benefit from this additional treatment escalation. Neither the dynamic deescalation nor the cocktail regimen appear to be dramatically inferior to the AcPSMA regimen of at least 100 kBq/kg.
The dynamic deescalation approach has been reported previously by Sathekge et al. (23). In a cohort of 17 chemotherapy-naïve patients, a fixed dose of 8 MBq of AcPSMA for the first cycle was administered, with consecutive deescalation in subsequent treatment cycles to 7, 6, or 4 MBq based on response to the previous treatment cycles. Grade 1 or 2 xerostomia was observed in all patients, but none was severe enough to lead to discontinuation of treatment. The week 8 PSA and best PSA responses of more than 50% were 76% (13/17) and 88% (15/17), respectively (23). The mOS could not be approximated because of the short follow-up of only 1 y (23). In a cohort of 73 men reported by the same group, xerostomia was seen in 85% of patients but never led to discontinuing treatment; a PSA response of more than 50% was observed in 70% of the patients, and mOS was 18 mo (24). Our results are similar with regard to tolerability, but only 53% of patients in the AcPSMA monotherapy group achieved a PSA response of more than 50%, which is inferior to the results from South Africa. However, this could be explained by the baseline characteristics. For example, lung, liver, and brain metastases were present in 3%, 5%, and 1% of the South African patients but 13%, 22%, and 2% of our patients (monotherapy group), respectively. Only 37% of the South African patients had previously received chemotherapy, but 85% of our patients had undergone such treatment.
So far, the largest study with dynamic deescalation of responding patients has been reported by Lawal et al. (25). Even though 32.1% of patients in this cohort presented with a superscan, hematologic tolerability was excellent and the superscan pattern was probably protective for salivary glands. Consequently, dose deescalation was done after 2 cycles of 8 MBq of AcPSMA. These results are confirmative to our experience that AcPSMA is well suited for patients with diffuse red marrow infiltration. It also supports the thesis that the initially proposed 100 kBq/kg tolerable dose might have been overestimated by the tumor sink effect.
A recent metaanalysis presented the most common side effects from AcPSMA with grade 1 or 2 xerostomia in 63.1% (89/141) of the evaluated patients (26). A complete overview of the available literature about AcPSMA monotherapy or combination therapy is provided in Table 2 (2,3,7–10,20–25,27–31).
Overview of Available Literature About AcPSMA Monotherapy or Combination Therapy
The LuPSMA VISION trial (19) presents the best standard of comparison to evaluate the antitumor activity of our treatment protocols. This trial recruited patients between May 2018 and August 2019; thus, these patients are not historical controls but were treated contemporaneously with our patients and consequently received nearly identical standards of care (Supplemental Table 1). The mOS of VISION patients was 15.3 mo, which is remarkably similar to our AcPSMA and LuPSMA cocktail group, with a mOS of 15 mo. Prospective phase 3 results should not be compared with less reliable real-world data; however, the number of deaths observed versus patients censored, as an important quality criterion for Kaplan–Meier statistics, is almost equal (Supplemental Table 1), and the objective parameter OS is independent of the clinical follow-up protocol used. However, some discrepancies between the patient cohorts exist. Several other experimental, off-label, or antiquated treatments had already been applied in advance of PSMA-TAT to our patients but were offered to VISION patients as additional treatment lines with potential antitumor activity not before their progression under LuPSMA trial medication (Supplemental Table 2). Bone marrow superscan was an exclusion criterion in the VISION trial but was present in a large fraction of our patients and was found to be significantly correlated with OS. In addition, 5%–16% of our patients would not have been candidates for the VISION trial because of low platelet count, and 12%–22% would not have been candidates because of low baseline hemoglobin. A recent literature review found a decreased efficacy of PSMA-TAT if patients were previously exposed to LuPSMA (32). However, 11% of our AcPSMA monotherapy patients and 7% of our AcPSMA and LuPSMA cocktail patients received 1 cycle and 26% of the AcPSMA monotherapy patients and 25% of the AcPSMA and LuPSMA cocktail patients received at least 2 cycles of β-PSMA–targeted radiopharmaceutical therapy before TAT. Altogether, we observed similar antitumor activity despite prognostically worse patients. This observation is in line with preclinical research comparing 177Lu- and 225Ac-labeled PSMA ligands, which reported a favorable absorbed dose distribution to tumor cell nuclei from PSMA-TAT, especially in micrometastases (33–35).
This evaluation has mentionable limitations. Because PSMA-TAT was offered on an individual patient basis, there was no prospective randomization, but deescalation approaches were offered as the physician’s choice. AcPSMA monotherapy was preferable and chosen in cases of diffuse-type organ infiltration, and the cocktail became standard once earlier-stage patients were scheduled by the referring urologists or oncologists. Real-world data are less stringent in the documentation of adverse events than pharmacovigilance monitoring in clinical trials; hence, they could not be evaluated systematically within this work. Because the urologist or oncologist chose the long-term follow-up (imaging and tumor markers) according to medical appropriateness, we were not able to obtain reliable radiologic progression-free survival data retrospectively.
CONCLUSION
Even deescalated treatment activities of the AcPSMA regimen or the AcPSMA and LuPSMA cocktail regimen present high antitumor activity in poor-prognosis prostate cancer. Considering the prognostic factors in the respective baseline profiles, the outcomes of both treatment regimens are well in line with previous AcPSMA studies. Treatment efficacy was similar to that reported for LuPSMA despite a worse initial situation. In contrast to previous high-dose regimens, none of the responding patients discontinued treatment because of xerostomia.
DISCLOSURE
Clemens Kratochwil and Uwe Haberkorn are coinventors of PSMA-617. Clemens Kratochwil, Uwe Haberkorn, Frederik Giesel, Frank Bruchertseifer, and Alfred Morgenstern are coinventors of an actinium-PSMA patent. Frederik Giesel and Uwe Haberkorn are coinventors of PSMA-1007. Frederik Giesel worked as scientific consultant for Telix. Clemens Kratochwil worked as scientific consultant for AAA/Novartis, Roche, and AdvanCell. Clemens Kratochwil, Uwe Haberkorn, and Frederik Giesel own shares of FAPI Holding. No other potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: Can the treatment activity of AcPSMA be reduced to improve tolerability compared with the current standard of 100 kBq/kgBW without losing too much antitumor activity?
PERTINENT FINDINGS: Despite worse prognostic baseline characteristics, mOS and PSA response rates are equivalent to VISION in patients with advanced-stage prostate cancer. None of the two tested approaches was obviously better than the other one.
IMPLICATIONS FOR PATIENT: Reduced-dose 225Ac and 225Ac/177Lu cocktail PSMA-617 therapies have strong antitumor activity and a tolerable trade-off of side effects.
Footnotes
Published online Jun. 6, 2024.
- © 2024 by the Society of Nuclear Medicine and Molecular Imaging.
REFERENCES
- Received for publication December 17, 2023.
- Accepted for publication April 25, 2024.