Visual Abstract
Abstract
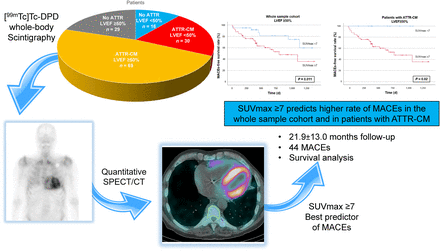
Quantitative 99mTc-3,3-diphosphono-1,2-propanodicarboxylic acid ([99mTc]Tc-DPD) SPECT may be used for risk-stratifying patients with amyloid transthyretin–related cardiomyopathy (ATTR-CM). We aimed to analyze the predictive value of quantitative [99mTc]Tc-DPD SPECT/CT in suspected and confirmed ATTR-CM according to different disease stages. Methods: The study enrolled consecutive patients with suspected ATTR-CM who were referred to a single tertiary center and underwent quantitative [99mTc]Tc-DPD SPECT/CT allowing SUVmax and SUVpeak analysis. Patients were divided into 2 groups according to left ventricular ejection fraction (LVEF) at baseline (i.e., ≥50% and <50%). Clinical, laboratory, and echocardiographic parameters and major adverse cardiac events (i.e., all-cause death, sustained ventricular tachyarrhythmia, hospitalization for heart failure, implantation of a cardioverter defibrillator) were investigated for any correlation with quantitative uptake values. Results: In total, 144 patients with suspected ATTR-CM were included in the study (98 with LVEF ≥ 50% and 46 with LVEF < 50%), of whom 99 were diagnosed with ATTR-CM (68.8%; 69 with LVEF ≥ 50% and 30 with LVEF < 50%). A myocardial SUVmax of at least 7 was predictive of major adverse cardiac events at 21.9 ± 13.0 mo of follow-up (hazard ratio, 2.875; 95% CI, 1.23–6.71; P = 0.015) in patients with suspected or confirmed ATTR-CM (global χ2 = 6.892, P = 0.02) and an LVEF of at least 50%. SUVmax was not predictive in patients with an LVEF of less than 50% and suspected or confirmed ATTR-CM. Conclusion: In patients with suspected or confirmed ATTR-CM and preserved LVEF, representing an early disease stage, quantitative [99mTc]Tc-DPD SPECT should be considered to improve early-stage risk stratification.
To diagnose amyloid transthyretin–related cardiomyopathy (ATTR-CM), 99mTc-3,3-diphosphono-1,2-propanodicarboxylic acid ([99mTc]Tc-DPD) scintigraphy and SPECT/CT have become established noninvasive methods (1–3). Although the visual interpretation according to Perugini score (4) is currently the clinical diagnostic standard, this visual grading failed to show an association with adverse clinical outcomes (5). However, the potential of semiquantitative [99mTc]Tc-pyrophosphate SPECT/CT to predict major adverse cardiac events (MACEs) has recently been described (6,7). Quantitative data in these studies were based on ratios between ventricular myocardial uptake and various structures of the body rather than on SUV, and there is a need to define whether SUV-based quantitative SPECT/CT can yield a robust prognostic value in patients with suspected ATTR-CM. Recent studies suggest that SUV may be proportional to the degree of active deposition of amyloid fibrils rather than to the amyloid burden within the myocardium (8–10). Hence, SUV may be hypothesized to be a prognostic marker whose importance is higher if assessed in the early stages of the disease, wherein a higher rate of amyloid fibril deposition could lead to a more rapid progression of ATTR-CM, potentially associated with an adverse prognosis. The aim of the current study was to evaluate the association of quantitative [99mTc]Tc-DPD SPECT/CT at baseline with outcomes in ATTR-CM patients with preserved and reduced left ventricular ejection fraction (LVEF), representing patients at earlier and more progressive disease stages, respectively.
MATERIALS AND METHODS
Patient Selection
In this retrospective study, we included all consecutive ATTR-CM patients from the Bern Cardiac Amyloidosis Registry who had been referred to the Department of Nuclear Medicine and the Department of Cardiology at Bern University Hospital between October 2019 and December 2022. All patients underwent [99mTc]Tc-DPD SPECT/CT for suspected ATTR-CM. Clinical, laboratory, and echocardiographic data were recorded both at the time of [99mTc]Tc-DPD SPECT/CT (baseline) and during follow-up according to a prespecified schedule. Patients were divided into 2 groups: a group with preserved left ventricular function (LVEF ≥ 50% as assessed by planar transthoracic echocardiography) and a group with impaired function (LVEF < 50%). The design of the study was approved by the local ethics committee. The study was registered with ClinicalTrials.gov (NCT04776824) and conducted in accordance with the Declaration of Helsinki.
[99mTc]Tc-DPD Scintigraphy
The imaging protocol has been published previously (2). In short, 674.19 ± 10.25 MBq of [99mTc]Tc-DPD were injected intravenously. Three hours after injection, whole-body planar imaging and subsequent SPECT/CT of the thorax were performed on a hybrid SPECT/CT system (Symbia Intevo; Siemens Medical Solutions AG). Images were reconstructed to a 256 × 256 matrix with a dedicated iterative algorithm (xSPECT/CT Quant; Siemens Medical Solutions AG), and low-dose CT was performed for attenuation correction.
Two independent readers graded the images using the previously validated visual score by Perugini et al. (4). Myocardial uptake on SPECT/CT images (SUVmax and SUVpeak) was automatically calculated for each patient using commercial software (SyngoVia Package; Siemens Medical Solutions AG) by generating an isocontour volume of interest with a 40% threshold of peak activity around the myocardial wall.
Follow-up
The following MACEs were considered for the time-to-first-event analysis: all-cause death, hospitalization due to heart failure, sustained ventricular tachyarrhythmia (≥30 s), or the implantation of a cardioverter defibrillator. During follow-up, changes in transthyretin-stabilizing therapy and heart failure medications were also recorded.
Statistical Analysis
Clinical, image-derived, and laboratory data were compared in the whole cohort and in the subgroup of patients with confirmed ATTR-CM between patients with preserved and impaired left ventricular function by means of the Mann–Whitney U test for continuous variables and the Fisher exact test for nominal variables. The optimal threshold for SUVmax to predict a MACE was assessed by means of receiver-operating-characteristic curve analysis with calculation of the Youden index. Cox regression analysis was used to correlate various clinical and imaging-derived parameters with MACEs (i.e., body mass index [BMI], N-terminal pro–B-type natriuretic peptide [NT-proBNP], left ventricular end-diastolic diameter [LVEDD], Perugini score, SUVmax and SUVpeak as a continuous variable, and SUVmax ≥ 7). If no MACEs were present in a group, the incident rate ratios were evaluated by calculating global χ2 by means of Poisson logistic regression. All these parameters were tested in a univariate analysis; since only an SUVmax of 7 or higher showed significance, no multivariate analysis including nonsignificant variables was performed. The treatment with tafamidis was not entered into the Cox regression analysis. The rate of MACEs was evaluated by means of Kaplan–Meyer curves with the log-rank test. To that end, the interval between baseline SPECT and onset of the first MACE was considered. The analysis was performed with SPSS (version 28.0.1.1; IBM) for Microsoft Windows. P values of less than 0.05 were considered statistically significant.
RESULTS
Patient Population
In total, 144 patients underwent quantitative [99mTc]Tc-DPD SPECT/CT for suspected ATTR-CM (mean age ± SD, 81.5 ± 5.8 y; 127 men [88.2%]). Of these, 99 (68.8%) were diagnosed with ATTR-CM on the basis of clinical, laboratory, and radiologic data. The characteristics of the patient populations are summarized in Table 1 (whole cohort with suspected ATTR-CM) and Table 2 (patients diagnosed with ATTR-CM).
Clinical Characteristics of Whole Patient Sample
Clinical Characteristics of Patients with Confirmed ATTR-CM
All patients with a Perugini score of at least 2 had a final diagnosis of ATTR-CM (65 with score 2, 27 with score 3). Among those with confirmed ATTR-CM, 7 had a Perugini score of 1. Of these, 2 patients had confirmed ATTR-CM due to evidence of increased extracellular volume (ECV) on subsequent cardiac MR (CMR); 4 patients, due to evidence of increased [99mTc]Tc-DPD uptake in the basal segments of the left ventriculum on SPECT images; and 1 patient, due to an SUVmax that was higher than the reference values reported in the literature (2).
Among patients diagnosed with ATTR-CM, 53 patients (53.5%) were started on transthyretin-stabilizing therapy (i.e., tafamidis, 61 mg administered once daily). In all patients with a Perugini score of 0, and in 2 patients with a Perugini score of 1, the diagnosis of ATTR-CM was rejected.
In the whole cohort (n = 144), patients with preserved LVEF had lower LVEDD and NT-proBNP levels than did subjects with impaired LVEF. Furthermore, they had a higher BMI and were on therapy more often with spironolactone and less often with sodium-glucose cotransporter 2 inhibitors and β-blockers (Table 1).
Similarly, among patients with confirmed ATTR-CM (n = 99), patients with preserved LVEF had a lower LVEDD than did patients with an LVEF of less than 50% but similar levels of NT-proBNP. They had a higher BMI and were under therapy more often with calcium channel antagonists and less often with sodium-glucose cotransporter 2 inhibitors and β-blockers.
Patients with an LVEF of at least 50% had a higher SUVmax and SUVpeak than did patients with an LVEF of less than 50%.
Follow-up
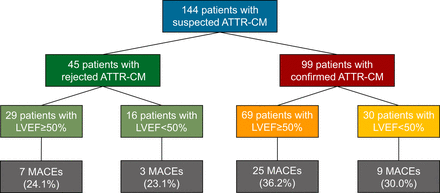
The median follow-up time was 21.9 ± 13.0 mo. A detailed flowchart with the main grouping is displayed in Figure 1. Of all patients, 98 presented with preserved LVEF (69 with ATTR-CM and 29 without), whereas 46 had impaired LVEF (30 with ATTR-CM and 16 without).
Study flowchart.
Considering the whole cohort, 44 MACEs occurred: 32 in patients with an LVEF of at least 50% (32.7%) and 12 in those with LVEF of less than 50% (26.1%, P = 0.28). Specifically, hospitalization for heart failure occurred in 17 patients with an LVEF of at least 50% (17.3%) and in 7 patients with an LVEF of less than 50% (15.2%, P = 0.30); sustained ventricular tachyarrhythmia occurred in 4 patients with an LVEF of at least 50% (4.1%) and in 1 patient with an LVEF of less than 50% (2.2%, P = 0.62); a cardioverter defibrillator was implanted in 5 patients with an LVEF of at least 50% (5.1%) and in 2 patients with an LVEF of less than 50% (4.4%, P = 0.57). Twenty-six patients eventually died (either as the first event or after another MACE): 16 patients with an LVEF of at least 50% (16.3%) and 10 patients with an LVEF of less than 50% (21.7%, P = 0.29).
In patients with ATTR-CM, 34 MACEs occurred: 25 in patients with an LVEF of at least 50% (25.3%) and 9 in those with an LVEF of less than 50% (9.1%, P = 0.36). Specifically, hospitalization for heart failure occurred in 16 patients with an LVEF of at least 50% (23.2%) and in 7 patients with an LVEF of less than 50% (23.3%, P = 0.28); sustained ventricular tachyarrhythmia occurred in 4 patients with an LVEF of at least 50% (5.8%) and in 1 patients with an LVEF of less than 50% (3.3%, P = 0.60); a cardioverter defibrillator was implanted in 4 patients with an LVEF of at least 50% (5.8%) and in 2 patients with an LVEF of less than 50% (6.6%, P = 0.51). Eighteen patients eventually died (either as the first event or after another MACE): 11 patients with an LVEF of at least 50% (15.9%) and 7 patients with an LVEF of less than 50% (23.3%, P = 0.27).
Summarizing, during follow-up, a total of 44 patients experienced a MACE (34 among patients with ATTR-CM [77.3%] and 10 in patients without [22.7%]).
Predictive Value of SPECT
At receiver-operating-characteristic curve analysis, an SUVmax of 7 was the best threshold to predict a MACE in patients with preserved LVEF (sensitivity, 72%; specificity, 49%). In the whole cohort, an SUVmax of at least 7 was the only parameter among the abovementioned clinical and imaging-derived ones that was associated with MACEs, but the association was present only in patients with an LVEF of at least 50% (hazard ratio, 2.875; P = 0.015). Conversely, SUVmax and SUVpeak as a continuous variable, LVEDD, BMI, and NT-proBNP did not have an association with MACEs (Table 3). When patients with confirmed ATTR-CM were considered, an SUVmax of at least 7 was again associated with MACEs only in patients with an LVEF of at least 50% (χ2 = 6.892, P = 0.009). SUVmax and SUVpeak as a continuous variable, LVEDD, BMI, and NT-proBNP did not predict MACEs (Table 4).
Hazard Ratio of Relevant Clinical and Imaging-Derived Parameters for Prediction of MACEs in Whole Patient Sample (n = 144)
Hazard Ratio of Relevant Clinical and Imaging-Derived Parameters for Prediction of MACEs in Patients with Confirmed ATTR-CM (n = 99)
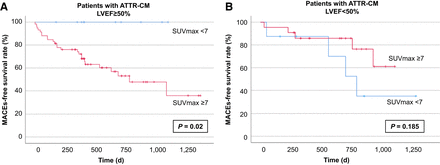
At Kaplan–Meyer analysis, an SUVmax of at least 7 identified among patients with an LVEF of at least 50% those at a shorter MACE-free survival, both in the whole cohort (P = 0.01, Fig. 2A) and in those with confirmed ATTR-CM (P = 0.02, Fig. 3A). The same held true after subdividing the latter on the basis of transthyretin-stabilizing therapy (n = 46, P = 0.01, Supplemental Fig. 1; supplemental materials are available at http://jnm.snmjournals.org).
Kaplan–Meyer curves highlighting predictive role of myocardial SUVmax ≥ 7 in whole cohort (n = 144). (A) In patients with preserved LVEF, SUVmax ≥ 7 as separator predicted higher rate of MACEs. (B) Conversely, no predictive role was seen in patients with already-impaired LVEF.
Kaplan–Meyer curves highlighting predictive role of myocardial SUVmax ≥ 7 in patients with confirmed ATTR-CM (n = 99). Similarly to what was observed for whole cohort, SUVmax ≥ 7 predicted higher rate of MACEs in patients with preserved LVEF (A) but not in patients with already-impaired LVEF (B).
Conversely, also at Kaplan–Meyer analysis, an SUVmax of at least 7 failed to predict MACE-free survival in patients with an LVEF of less than 50%, either in the whole cohort (P = 0.27, Fig. 2B) or in the those with confirmed ATTR-CM (P = 0.19, Fig. 3B).
A tertile analysis based on SUVmax in patients with ATTR-CM (first tertile, <9.76; second tertile, ≥9.76% and <15.95; third tertile, ≥15.95) showed a clear tendency toward worse outcomes for patients with higher SUVmax if LVEF was at least 50% (P = 0.06), whereas no differences were shown in patients with an LVEF of less than 50% (P = 0.46, Supplemental Fig. 2). After patients with a Perugini score of 1 were excluded, an SUVmax of at least 7 was still predictive of a worse outcome in patients with an LVEF of at least 50%, both in the whole population (P = 0.037) and in the subgroup of patients with confirmed ATTR-CM (P = 0.039, Supplemental Fig. 3). Again, no significance was found in patients with impaired LVEF (P = 0.401 and 0.291, respectively).
Visual analysis failed to yield prognostic value: Perugini score showed no association with the onset of MACEs in patients with and without preserved LVEF, either in the whole cohort (Table 3) or in patients with confirmed ATTR-CM (Table 4).
DISCUSSION
We demonstrated for the first time, to our knowledge, the value of quantitative [99mTc]Tc-DPD SPECT/CT in the prediction of MACEs in patients with suspected and, most importantly, confirmed ATTR-CM and preserved LVEF, representing an early disease stage. This association was not found in patients with reduced LVEF, representing later disease stages. Although previous work (6,7) used semiquantitative, normalized ratios, we here report a robust predictive value for myocardial SUV. This aspect provides novelty in that it supports the concept that the activity within the heart itself (and not the relative activity compared with bone or soft tissue) reflects a pathophysiologic mechanism linked to disease progression.
In this regard, the exact significance of different degrees of [99mTc]Tc-DPD uptake within the myocardium deserves a detailed discussion.
Perugini visual score failed to show prognostic value in patients with ATTR-CM (5). To account for the lack of prognostic value, it was hypothesized that visual interpretation does not accurately reflect the amount of amyloid burden in the myocardium. This latter would be predictive of a different outcome, but Perugini score may indicate only the presence of amyloid deposition, without information on the amyloid burden (2). Although quantification with SPECT/CT was expected to be more accurate in this regard, previous studies on patients at different stages of ATTR-CM showed only limited prognostic value and used normalized values only, thus raising doubts on the linear correlation between [99mTc]Tc-DPD uptake and amyloid burden (6,7). Conversely, studies capitalizing on CMR imaging showed that increased ECV correlates well with amyloid burden within the myocardium and allows for risk stratification (11,12). Hence, there is a clear discrepancy between the prognostic value of quantitative SPECT and CMR, which is probably related to the different target.
Recently published papers (8–10) show that myocardial [99mTc]Tc-DPD uptake decreases after therapy with tafamidis. If we assumed that the uptake is proportional to the amyloid burden, these data would be counterintuitive, as tafamidis acts essentially by preventing further deposition of amyloid fibrils rather than degrading them. Hence, it is conceivable that [99mTc]Tc-DPD uptake is proportional not to amyloid burden but rather to the degree of active deposition of amyloid fibrils within the myocardium, which can be lowered by transthyretin-targeting therapies. Of note, this concept is consistent with what is observed in other fields of cardiovascular imaging, wherein nuclear medicine techniques are preferred modalities to distinguish between the active phase and the chronic phase of the disease (13). The discrepancy between SPECT and CMR is further evidenced by a recent CMR study in which stabilization of ECV after treatment with tafamidis was reported, which may indicate that ECV is proportional to amyloid burden and not to its active deposition (14).
Taken together, these observations suggest that in an early stage of ATTR-CM, the amyloid burden may not be high but deposition of amyloid fibrils can already be rapid. In this setting, it is conceivable that assessment of the activity of such deposition by evaluating [99mTc]Tc-DPD can yield prognostic value, as it would be an indicator of the more or less rapid evolution of the disease (15). For that reason, we investigated for the first time, to our knowledge, specifically patients with preserved LVEF, which would indicate an earlier stage of ATTR-CM.
Consistent with previous reports, our study showed that visual Perugini scoring is not predictive of MACEs in ATTR-CM patients. Yet, SUV-based quantitative SPECT showed prognostic value, and in this regard, our study provided novelty regarding another 2 major aspects.
First, we demonstrated that patients with preserved or impaired LVEF have a similar rate of MACEs, but differences become significant if patients are further stratified according to their [99mTc]Tc-DPD uptake. This provides more evidence that the degree of active deposition of the amyloid fibrils may be the major determinant of the rate of MACEs in patients with ATTR-CM and supports the concept that quantitative [99mTc]Tc-DPD SPECT/CT allows for an in vivo assessment of this activity.
Second, the same prognostic value pertained also to patients with suspected ATTR-CM. To date, the clinical diagnosis of ATTR-CM relies on a combination of clinical, echocardiographic, and radiologic findings, and DPD scintigraphy represents a cornerstone in confirming or rejecting the diagnosis (16). In our population, some of the patients with suspected ATTR-CM had clinical and echocardiographic signs of the disease, but the diagnosis was eventually rejected because [99mTc]Tc-DPD SPECT had negative findings. In this regard, how to interpret a Perugini score of 1 (i.e., detectable myocardial uptake below bone activity) is contentious, and a score of 1 often does not allow for a definite diagnosis. But a proportion of our patients with confirmed ATTR-CM (n = 7) had detectable cardiac [99mTc]Tc-DPD uptake visually below bone activity. Considering the robust prognostic value in our whole cohort and in the cohort with patients diagnosed with ATTR-CM, there may be a rationale to consider patients with a Perugini score of 1 as patients with ATTR-CM in a very early stage or with a weak deposition of fibrils, which may, however, accelerate at a certain time point. This aspect has an evident impact on the management of patients with suspected ATTR-CM and may suggest the need for further SPECT imaging at shorter intervals, which will need to be defined.
This study had some limitations. Its retrospective nature prevented us from recruiting patients with standardized therapy. However, the patient cohort was large enough to allow for a subanalysis in patients not under transthyretin-stabilizing therapy. Second, this was a registry study and not a specific powered study. The fact that a clearly worse outcome was present in patients with high myocardial uptake and preserved LVEF strengthens the confidence with which [99mTc]Tc-DPD uptake can be considered an indicator of active deposition of amyloid fibrils—the more active the deposition, the more rapid the development of the disease—whereas the impact of the rate of amyloid fibril deposition is not expected to play a major role if a large amyloid burden is already present. Although further and larger studies are warranted to validate this concept, the data of our study constitute a fundamental basis for further research. Third, we used an already-validated method for quantitation relying on a proprietary hardware–software system; therefore, our results may not fit the needs of clinical centers using different quantification software, and separate validations are needed. Nevertheless, the fact that a clear predictive role was demonstrated for MACEs in patients with preserved LVEF supports the applicability of the quantitative approach in clinical practice for risk stratification. In this regard, it may be suggested that patients with an SUVmax of at least 7 on baseline SPECT/CT be considered for tafamidis therapy. Fourth, the choice of LVEF as a marker of a more advanced stage of ATTR-CM does not reflect the current recommendations, which suggest the use of NT-proBNP and glomerular filtration rate (eGFR) (17). Also, other echo-derived parameters such as global longitudinal strain have been suggested as indicative of a more advanced stage of the disease (11). Although this choice reflects the retrospective nature of the present study (data on NT-proBNP, eGFR, and global longitudinal strain were not all available in all patients), there still is a rationale to consider LVEF as a marker of advanced disease. A paper by Knight et al. (11) showed an inverse correlation between LVEF and ECV, with impaired left ventricular function in patients with higher ECV. Hence, it is conceivable that patients with impaired LVEF in our cohort were also those in a more advanced stage. Further prospective studies are warranted to support our data.
Finally, we observed that patients with impaired LVEF had a higher LVEDD, suggesting some degree of dilation, which can be seen at the end stage of the disease but is not typical. In our cohort, we could not rule out that a subset of those patients with an LVEF of less than 50% had a concomitant pathologic condition causing left ventricular dilation. The presence of a possible competing risk due an undiagnosed condition may be responsible for the lack of prognostic value of [99mTc]Tc-DPD uptake in these patients. These observations may be a starting point for further studies featuring patients with ATTR-CM and impaired LVEF.
CONCLUSION
In patients with suspected or confirmed ATTR-CM and preserved LVEF, representing an early disease stage, a myocardial SUV of at least 7 is a predictive marker for MACEs. Quantitative [99mTc]Tc-DPD SPECT should be considered for improved early-stage risk stratification of patients with ATTR-CM.
DISCLOSURE
This study (NCT04776824) was supported by Pfizer and the GAMBIT Foundation and was based on the database of the Bern Cardiac Amyloidosis Registry (B-CARE). Federico Caobelli is supported by a research grant by Siemens Healthineers and receives speaker honoraria from Bracco AG and Pfizer AG for matters not related to the present paper. Christoph Gräni receives funding from the Swiss National Science Foundation, InnoSuisse, the CAIM Foundation, and the Novartis Biomedical Research Foundation, outside the submitted work. Axel Rominger has received research support and speaker honoraria from Siemens. Stephan Dobner reports a research grant for B-CARE (NCT04776824) on behalf of the institution (Inselspital Bern) from Pfizer and travel grants and speaker fees from Alnylam and Boehringer Ingelheim and Pfizer. No other potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: Is quantitative [99mTc]Tc-DPD SPECT/CT suitable to identify patients at increased risk of cardiac events among those in an earlier stage of ATTR-CM?
PERTINENT FINDINGS: In a cohort study including 144 patients (99 with ATTR-CM), an [99mTc]Tc-DPD SUVmax of 7 or higher identified patients with preserved left ventricular function at increased risk of cardiac events. Conversely, patients with impaired function could not be risk-stratified by SPECT/CT, thus highlighting the fact that the latter are at a later stage of disease, wherein the impact of a more active deposition of amyloid fibrils is not expected to play a major role in regard to the outcome.
IMPLICATIONS FOR PATIENT CARE: Identifying patients with still-preserved left ventricular function at a higher risk of disease progression may drive the decision to choose a more aggressive therapy, such as transthyretin stabilizers.
Footnotes
↵* Contributed equally to this work.
Published online May 9, 2024.
- © 2024 by the Society of Nuclear Medicine and Molecular Imaging.
REFERENCES
- Received for publication October 24, 2023.
- Revision received April 3, 2024.