Visual Abstract
Abstract
In contemporary oncologic diagnostics, molecular imaging modalities are pivotal for precise local and metastatic staging. Recent studies identified fibroblast activation protein as a promising target for molecular imaging across various malignancies. Therefore, we aimed to systematically evaluate the current literature on the utility of fibroblast activation protein inhibitor (FAPI) PET/CT for staging patients with genitourinary malignancies. Methods: A systematic Embase and Medline search was conducted, according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) process, on August 1, 2023. Relevant publications reporting on the diagnostic value of FAPI PET/CT in genitourinary malignancies were identified and included. Studies were critically reviewed using a modified version of a tool for quality appraisal of case reports. Study results were summarized using a narrative approach. Results: We included 22 retrospective studies with a cumulative total of 69 patients, focusing on prostate cancer, urothelial carcinoma of the bladder and of the upper urinary tract, renal cell carcinoma, and testicular cancer. FAPI PET/CT was able to visualize both local and metastatic disease, including challenging cases such as prostate-specific membrane antigen (PSMA)–negative prostate cancer. Compared with radiolabeled 18F-FDG and PSMA PET/CT, FAPI PET/CT showed heterogeneous performance. In selected cases, FAPI PET/CT demonstrated superior tumor visualization (i.e., better tumor-to-background ratios and visualization of small tumors or metastatic deposits visible in no other way) over 18F-FDG PET/CT in detecting local or metastatic disease, whereas comparisons with PSMA PET/CT showed both superior and inferior performances. Challenges in FAPI PET/CT arise from physiologic urinary excretion of most FAPI radiotracers, hindering primary-lesion visualization in the bladder and upper urinary tract, despite generally providing high tumor-to-background ratios. Conclusion: The current findings suggest that FAPI PET/CT may hold promise as a future tool to aid clinicians in detecting genitourinary malignancies. Given the substantial heterogeneity among the included studies and the limited number of patients, caution in interpreting these findings is warranted. Subsequent prospective and comparative investigations are anticipated to delve more deeply into this innovative imaging modality and elucidate its role in clinical practice.
- prostate cancer
- urothelial carcinoma
- renal cell carcinoma
- testicular cancer
- metastatic screening
- FAPI PET/CT
Accurate staging of genitourinary malignancies is crucial for optimizing treatment planning and patient outcomes (1–6). Recent advances in molecular imaging modalities, such as radiolabeled 18F-FDG and prostate-specific membrane antigen (PSMA) PET/CT, have significantly advanced the staging of genitourinary malignancies, compared with conventional imaging methods (7–9). These molecular imaging modalities excel at detecting metastatic disease even in anatomically nonanomalous structures. However, challenges persist. For instance, current molecular imaging modalities often struggle to detect all metastatic lesions, demonstrating the relatively low sensitivity of 18F-FDG and PSMA PET/CT, false positives due to physiologic uptake in tissues not within the region of interest or (postoperative) inflammation, and the occasional absence of PSMA avidity. Thus, exploring alternative imaging modalities is essential to enhance staging accuracy and guide therapeutic decisions (10–12).
Malignancies, including genitourinary cancers, comprise diverse cell types and not just autonomic neoplastic cells (Fig. 1) (13). Activated fibroblasts or myofibroblasts, also known as cancer-associated fibroblasts, are abundant in the tumor microenvironment and possess various significant functions, such as promotion of tumor growth, cell invasion and metastasis, angiogenesis, and regulation of immune response (14). Distinguishing themselves from normal fibroblasts, most cancer-associated fibroblasts exhibit remarkable overexpression of the fibroblast activation protein (FAP), making them an appealing target for noninvasive molecular imaging techniques (15,16). Recently, a quinoline-based FAP inhibitor (FAPI) was identified, which binds selectively to this epitope, and after being labeled with positron-emitting isotopes, the tumor microenvironment could be visualized in vivo using PET/CT imaging (17).
Graphic illustration of tumor microenvironment with cancer-associated fibroblasts (CAFs) and their overexpression of FAP. (Created with BioRender.com.)
Several publications have shown that FAPI PET/CT yields strong positive signals in various malignancies, suggesting its potential as a highly cancer-specific imaging modality that may overcome the limitations of 18F-FDG and PSMA PET/CT (18–20). In this review, we systematically assess the current literature regarding the diagnostic value of FAPI PET/CT for genitourinary malignancies.
MATERIALS AND METHODS
This systematic review was registered in the International Prospective Register of Systematic Reviews database on July 9, 2023 (CRD42023443837). The subsequent methodology followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (21).
Search Strategy
A systematic Embase and Medline search was conducted on August 1, 2023. The initial search included some of the following key terms: “Fibroblast Activation Protein Inhibitor” OR “FAPI” AND (“Bladder Cancer” OR “Prostate Cancer” OR “Penile Cancer” OR “Renal Cancer” OR “Urachal Cancer” OR “Testicular Cancer” OR “Urethral Cancer” OR “Upper Tract Urinary Cancer”). The complete free-text search terms are attached as supplemental material (supplemental materials are available at http://jnm.snmjournals.org). For searches, publication dates were limited to the preceding 10 y, because the first FAPI tracer was introduced in the preceding decade. No other limits were applied.
Inclusion and Exclusion Criteria
Studies were included for review if they explored the diagnostic value of FAPI PET/CT in patients with genitourinary malignancies (i.e., urothelial carcinoma of the bladder and upper urinary tract, prostate cancer [PCa], penile cancer, renal cell carcinoma [RCC], mucinous urachal adenocarcinoma, and testicular cancer). Hence, in accordance with the PRISMA statement, we used a strategy considering population, intervention, comparison, and outcome elements to select studies that report on the diagnostic value of FAPI PET/CT in patients with genitourinary malignancies, comparing it with established molecular imaging modalities. Publications were excluded if they were not written in English, related to nonclinical results, or were published as letters to the editor, editorials, study protocols, or commentaries or were in the gray literature. Finally, if multiple studies reported results from overlapping cohorts, only the most recent publication was retrieved.
Systematic Review Process
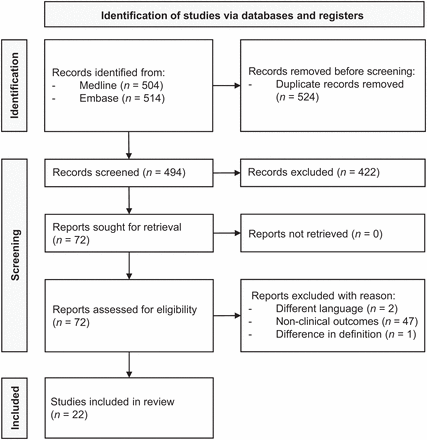
The abstract and full-text screening and the subsequent data extraction were performed by 2 reviewers independently. Discrepancies between reviewers were resolved by consensus. Relevant publication reference lists were screened manually to identify further studies. The PRISMA flowchart describing the systematic review process, with the numbers of papers identified and included or excluded at each stage, is presented in Figure 2.
PRISMA flow diagram showing outcome of searches and selection of full studies included in review.
Data Extraction
Information on study characteristics was extracted from all included studies by 2 of the authors, who subsequently cross-checked these to ensure their accuracy. A standardized data extraction form was created a priori to collect article information (first author name, year of publication, study design, and number of participants), population characteristics of interest (tumor type, histologic subtype, and disease stage), and imaging details (radiotracer and comparative imaging modality) and findings. Additionally, comparative information with other imaging modalities or histopathologic specimens was likewise collected, ensuring a comprehensive evaluation of the diagnostic performance of FAPI PET/CT in relation to alternative imaging techniques and pathologic findings.
Quality Appraisal
Assuming that identified reports would involve primarily nonrandomized studies, such as cohort studies and case reports, identified studies were critically reviewed using a modified version of a tool for quality appraisal of case reports (22). The assessment determined 4 items: whether the patient was described adequately (i.e., main complaint, history, clinical and laboratory evaluations, treatments), whether an accurate diagnosis was provided (i.e., valid and reliable outcome measures were used), whether convincing evidence in support of the diagnosis was presented (i.e., according to the criteria for diagnosis of antiphospholipid syndrome or catastrophic antiphospholipid syndrome, or describing the evidence for diagnosis), and whether alternate explanations were considered and refuted (differential diagnosis was illustrated and scientifically excluded, or underlying possible mechanisms that could explain the finding were addressed). Possible item ratings were yes, partially, or no.
Statistical Analysis
Studies were assessed using a narrative synthesis of included studies and descriptive statistics to summarize the data on extracted baseline characteristics. The diagnostic value of FAPI PET/CT was described with regard to its ability to detect metastatic disease, and it was compared with current molecular imaging modalities if possible. A metaanalysis was not performed, because of the expected heterogeneity of the included studies.
RESULTS
The first reviewer identified 65 potentially eligible studies, and the second reviewer identified 75 potential studies, resulting in an 87% agreement rate. Ultimately, 22 studies were eligible for inclusion, all of which were retrospective and with a high or unclear risk of bias (19,23–43). Supplemental Table 1 summarizes the quality appraisal. All 22 studies focused on assessing the diagnostic value of FAPI PET/CT in various genitourinary malignancies, including PCa (n = 12), urothelial carcinoma of the bladder and upper urinary tract (n = 4), RCC (n = 6), and testicular cancer (n = 1). The systematic review encompassed a cumulative total of 69 patients. Table 1 presents article information, population characteristics, and molecular imaging details stratified by tumor type.
Included Studies Using FAPI PET/CT in Various Genitourinary Malignancies
FAPI PET/CT in PCa
Of the 69 patients assessed, 33 (48%) underwent FAPI PET/CT for staging PCa. Most FAPI PET/CT scans were performed on a known-metastatic population with a history of prior treatment (19,29,34,37,40–42). FAPI PET/CT was able to visualize metastatic PCa lesions (nodal, skeletal, or visceral) because of the generally high tracer uptake and minimal background activity of FAPI radiotracers, resulting in high tumor-to-background ratios (TBRs). Similarly, in newly diagnosed PCa patients, the primary tumor exhibited excellent visualization, with high radiotracer uptake (23,26,28,31,32).
In comparison to 18F-FDG and PSMA PET/CT, FAPI PET/CT demonstrated a variable diagnostic performance, including instances of both higher and lower diagnostic yields, such as numbers of metastases (23,26,29,34,40–42). Improved diagnostic outcomes (i.e., higher diagnostic yield) were particularly observed in cases with more advanced stages of PCa (37,40–42). Interestingly, several cases demonstrated the ability of FAPI PET/CT to visualize local and metastatic disease in patients with PSMA-negative tumors (26,37,40,42).
Given the focus on an established metastatic population, histopathologic confirmation was infrequently pursued. In studies with histopathologic evidence, this confirmation was limited to the primary tumor (23,26,29). Hence, a direct comparison of FAPI PET/CT with conventional molecular imaging using histopathology as a reference standard was generally not possible in the included cases.
FAPI PET/CT in Urothelial Carcinoma of the Bladder and Upper Urinary Tract
FAPI PET/CT was performed on 29 patients to stage urothelial carcinoma of the bladder (n = 22) and upper urinary tract (n = 7). FAPI PET/CT focused primarily on newly diagnosed patients, constituting 20 of 29 cases (25,30,33,43). The notably high TBRs in FAPI PET/CT generally facilitated excellent visualization of metastatic lesions (25,30,33,43). However, because of the elevated background signal resulting from renal and urinary excretion of most FAPI radiotracers, primary tumors in the bladder and upper urinary tract were often not visible (25,30,33). Similarly, lymph nodes near the urinary tract posed a similar challenge due to overlapping urinary activity (30).
Compared with 18F-FDG PET/CT, FAPI PET/CT has shown enhanced diagnostic value in included cases. FAPI PET/CT yielded an increased number of identified lesions compared with 18F-FDG PET/CT and correctly reclassified suggestive (reactive) lesions as nonsuggestive (25,30,43). Histopathologic confirmation, however, remained limited in these studies, with the report by Unterrainer et al. being the only one with histopathologic confirmation (30). Their findings showed that FAPI PET/CT could accurately identify lymph node metastases; only 1 histopathologically confirmed lymph node was missed.
FAPI PET/CT in RCC
Of the evaluated patients, 6 underwent FAPI PET/CT for RCC (19,24,27,35,38,39). Noteworthy is the fact that these RCC cases encompassed different subtypes, including sarcomatoid, chromophobe, papillary, and clear cell. Both newly diagnosed and known-metastatic patient populations were studied, with 4 of 6 cases falling within the latter category. Despite the report by Kratochwil et al. (19) of low tumoral tracer uptake in RCC, other investigators reported excellent visualization of both primary and metastatic lesions. Notably, FAPI PET/CT detected a solitary choroid plexus metastasis because of lack of physiologic cerebral uptake with FAPI radiotracers (39).
In comparison to 18F-FDG PET/CT, FAPI PET/CT detected an equal or greater number of metastatic lesions and provided enhanced delineation of these lesions due to the higher TBRs of FAPI radiotracers. Additionally, if the primary tumor was not visualized on 18F-FDG PET/CT, FAPI PET/CT successfully revealed the primary lesion (27). Dong et al., Pang et al., and Yang et al. provided histopathologic confirmation for both metastatic and primary lesions, with FAPI-positive lesions consistently confirmed to harbor metastatic disease (24,27,39).
FAPI PET/CT in Testicular Cancer
The diagnostic value of FAPI PET/CT in testicular cancer has been studied only once, involving a patient diagnosed with a metastatic mixed germ cell tumor (36). In contrast to previously mentioned genitourinary malignancies, this study demonstrated only mild tumoral tracer uptake. Nevertheless, the uptake was increased with FAPI PET/CT compared with 18F-FDG PET/CT. Regrettably, histopathologic confirmation of the metastatic lesions was not provided.
DISCUSSION
Molecular imaging techniques have demonstrated added diagnostic value for various genitourinary malignancies, such as PSMA PET/CT for PCa and 18F-FDG PET/CT for urothelial carcinoma of the bladder and upper urinary tract (7,44,45). Nevertheless, the pursuit of more sensitive and specific imaging modalities continues, as an ideal imaging modality for metastatic screening must combine near-perfect specificity with maximized sensitivity (1–6). This systematic review summarizes the existing evidence regarding the diagnostic value of FAPI PET/CT across genitourinary malignancies. Despite an adequate number of identified studies, limitations in the patient cohorts staged with this novel imaging modality within our review, along with the overall low quality of studies, preclude definitive conclusions regarding its value in staging both local and metastatic disease. Nevertheless, this comprehensive review offers valuable insights for future research.
It is evident that research on FAPI PET/CT imaging is extremely limited and lacks high-level data. However, in contrast to genitourinary malignancies, for which such data are lacking, research on the diagnostic value of FAPI PET/CT has significantly advanced in other types of malignancies (46–52). Particularly, FAPI PET/CT has displayed significant potential in gastrointestinal malignancies such as colorectal, gastric, pancreatic, and liver cancers, as well as in lung cancer. Its superior performance compared with 18F-FDG PET/CT in identifying primary tumors, local recurrences, lymph node involvement, and diverse metastatic lesions highlights its value in clinical management within these oncologic domains. In contrast, its management impact in genitourinary malignancies is yet to be determined. As research continues, it is worth noting that ongoing trials, as registered on ClinicalTrials.gov, are also extending their investigations to encompass a broader spectrum of malignancies, such as oral, breast, ovarian, prostate, and bladder cancers. These ongoing investigations are poised to provide a more comprehensive understanding of the diagnostic capabilities of FAPI PET/CT, bridging the gap in research within the genitourinary domain and enhancing the broader landscape of its applications in oncology as a whole.
Similar to its application in these other malignancies, FAPI PET/CT has demonstrated potential advantages and disadvantages in genitourinary malignancies (19,33,53–55). One reported advantage of FAPI PET/CT in genitourinary malignancies is its ability to achieve a high TBR, facilitating excellent tumor visualization, precise delineation, and accurate localization of metastatic disease. Particularly advantageous is the minimal accumulation of FAPI radiotracers in the abdomen and intestinal tract, improving detection of metastatic disease in these regions (i.e., peritoneal metastases) (33,43,54–58). FAPI radiotracers, however, come in various forms (58). Although their biodistribution is generally comparable, variations can be observed in physiologic uptake and tumor-to-blood ratios, among others. Head-to-head comparisons between different FAPI radiotracers are currently still lacking, highlighting the need for further research in this area. Challenges generally associated with all FAPI radiotracers relate to urinary excretion, a characteristic shared with 18F-FDG and certain PSMA radiotracers. This excretion hampers the visualization of primary tumors in the bladder and upper urinary tract (30,56,57,59). Nevertheless, a promising aspect of FAPI radiotracers is their rapid and high tumoral tracer uptake, often detectable within 10 min after tracer administration (33). This rapid and intense FAPI uptake suggests a potential solution: enabling early PET image acquisition even before urinary excretion has occurred, addressing the challenge posed by urinary activity (33).
Comparing FAPI PET/CT with PSMA and 18F-FDG PET/CT in genitourinary malignancies provides significant insights. Unlike 18F-FDG, both PSMA and FAPI specifically target overexpressed molecules in malignancies. FAPI PET/CT’s distinctive feature of avoiding metabolic activity assessment potentially enhances visualization in challenging cases, such as cerebral metastases of RCC, and may enable differentiation of reactive from metastatic lymph nodes (25,30,39,60), unlike 18F-FDG PET/CT. However, FAPI PET/CT exhibited varying diagnostic value compared with PSMA PET/CT across the included reports, indicating potential dependence on the varying FAP expression in PCa (37,61). FAP expression significantly increases with disease progression, reaching its lowest detection in benign and treatment-naïve patients (37). This indicates a potentially greater diagnostic value of FAPI PET/CT in more advanced disease stages. Moreover, FAPI PET/CT demonstrated radiotracer uptake in both local and metastatic lesions of PSMA-negative PCa patients, suggesting its potential as an alternative diagnostic tool in this specific subset (26,37,40,42). However, we also found that FAPI PET/CT is not devoid of false-positive findings. Although FAP overexpression is common in malignancies, it can also be present during extracellular matrix remodeling, wound healing, and other benign conditions, such as arthritis, fibrosis, and ischemic heart tissue after a myocardial infarction (15,31), as well as atherosclerosis, autoimmune disease, and metabolic diseases. Given FAP’s role in tissue remodeling and expression on activated fibroblasts of scarring tissue, FAP expression is related to diseases of uncontrolled scarring (fibrosis). FAP has been reported elevated in fibrotic conditions involving the liver, lung, and colon (e.g., cirrhosis, idiopathic pulmonary fibrosis, keloid formation, Crohn disease). Hence, false negatives and false positives remain possible with FAPI PET/CT; however, their rates cannot be reliably assessed in this review because of the limited number of patients and lack of histopathologic confirmation.
FAPI PET/CT has shown promising initial results in genitourinary malignancies; however, a comprehensive assessment of the full spectrum of these malignancies was not achieved. This systematic review did not encompass research on specific rare cancers, including mucinous urachal adenocarcinoma, urethral carcinoma, and penile squamous cell carcinoma. Studies on the application of FAPI PET/CT within these rare genitourinary malignancies were either not identified or ineligible for inclusion. Noteworthy findings concerning the application of FAPI PET/CT in penile squamous cell carcinoma, however, have been reported in a conference abstract by Eismann et al. (62), who reported the successful identification of all histopathologically confirmed lymph node metastases by FAPI PET/CT. Interestingly, in half of their patients, focal uptake of FAPI was observed in primary lesions, whereas in the remaining half, visualization of primary lesions was hindered by increased urinary activity—a pattern consistent with previous observations (30,56,57). Refined imaging protocols (i.e., omission of furosemide and micturition before the scan) might improve the visualization of primary lesions, although these results are still awaited (62).
Signifying substantial promise in diagnostics, targeting of FAP offers not only diagnostic potential but also the prospect of theranostic applications. The high TBR and long retention allow for potential therapeutic use by labeling FAPI with α- or β-emitting isotopes, yielding a potent therapeutic agent (63). Currently, early-stage clinical exploration focuses primarily on assessing the feasibility, biodistribution, and dosimetry of FAPI radioligand therapy in various malignancies, demonstrating good tolerability and acceptable side effects (64). Although data on FAPI radioligand therapy in genitourinary malignancies are limited, there is growing recognition of its potential benefits. This is particularly notable for specific patients exhibiting inadequate responses to current management options or for individuals for whom existing therapeutic alternatives are unsuitable because of factors such as insignificant PSMA expression (28,30,33–35,40–42).
Because of the novelty of this imaging modality, our review is constrained by the retrospective nature of all included studies, predominantly consisting of case reports, case series, and retrospective cohort studies. Consequently, inherent limitations such as selection bias and publication bias are present. The studies we incorporated displayed a significant or unclear risk of bias and lacked a standardized approach to reporting outcomes. Moreover, because of the lack of histopathologic confirmation or follow-up imaging, FAPI PET/CT findings have yet to be validated. Furthermore, the overall number of patients investigated was small, and significant heterogeneity was observed across disease types, clinical settings, and FAPI PET/CT (i.e., radiotracer use and acquisition techniques), making it impossible to draw any conclusion or make any statement on the sensitivity of FAPI PET/CT. These findings underscore the limitations of the current body of research and emphasize the imperative for meticulous and comprehensive exploration and documentation of this imaging modality in the context of genitourinary malignancies.
CONCLUSION
This systematic review synthesizes the existing evidence regarding the diagnostic value of FAPI PET/CT in various genitourinary malignancies, emphasizing the limited availability and low quality of data. Current preliminary research hints at the potential of FAPI PET/CT to effectively visualize both local and metastatic disease in selected patients with various genitourinary malignancies. However, given the scarcity of comprehensive data, the use of FAPI PET/CT seems most appropriate for experimental settings until its potential in clinical practice is established.
DISCLOSURE
No potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: What is the current evidence regarding the diagnostic value of FAPI PET/CT for genitourinary malignancies?
PERTINENT FINDINGS: This systematic review, emphasizing the limited availability and low quality of data, hints at the potential of FAPI PET/CT to effectively visualize both local and metastatic disease in patients with various genitourinary malignancies. Notably, FAPI PET/CT shows potential in distinguishing between reactive and metastatic lymph nodes and in visualizing local and metastatic disease in patients with PSMA-negative tumors.
IMPLICATIONS FOR PATIENT CARE: Metastatic screening using FAPI PET/CT could potentially aid in more accurate staging of genitourinary malignancies and thereby optimize treatment planning and patient outcomes.
Footnotes
Published online Apr. 18, 2024.
- © 2024 by the Society of Nuclear Medicine and Molecular Imaging.
REFERENCES
- Received for publication December 18, 2023.
- Revision received March 4, 2024.