Visual Abstract
Abstract
Metastasis-directed therapy has the potential to improve progression-free and overall survival in oligometastatic disease (OMD). For breast cancer, however, randomized trials have failed so far to confirm this finding. Because the concept of metastasis-directed therapy in OMD is highly dependent on the accuracy of the imaging modality, we aimed to assess the impact of 18F-FDG PET/CT on the definition of OMD in breast cancer patients. Methods: Eighty patients with a total of 150 18F-FDG PET/CT images (between October 2006 and January 2022) were enrolled in this retrospective study at the Technical University of Munich. The inclusion criteria were OMD, defined as 1–5 distant metastases, at the time of 18F-FDG PET/CT. For the current study, we systemically compared the metastatic pattern on 18F-FDG PET/CT with conventional CT. Results: At the time of 18F-FDG PET/CT, 21.3% of patients (n = 32) had a first-time diagnosis of metastatic disease, 40.7% (n = 61) had a previous history of OMD, and 38% (n = 57) had a previous history of polymetastatic disease. In 45.3% of cases, the imaging modality (18F-FDG PET/CT vs. conventional CT) had an impact on the assessment of whether OMD was present. An identical metastatic pattern was observed in only 32% of cases.18F-FDG PET/CT detected additional metastases in 33.3% of cases, mostly in the nonregional lymph node system. Conclusion: The use of 18F-FDG PET/CT had a substantial impact on the definition of OMD and detection of metastatic pattern in breast cancer. Our results emphasize the importance of establishing a standardized definition for imaging modalities in future trials and clinical practices related to metastasis-directed therapy in breast cancer patients.
Oligometastatic disease (OMD) can be considered an intermediate stage between a locally limited tumor and disseminated metastatic disease, as postulated by Hellman and Weichselbaum in 1995. They hypothesized that local treatment of all identified metastases in OMD may prevent progression of the disease (1). Most recently, several randomized trials have supported this hypothesis with promising clinical results (2–4).
The SABR-COMET trial showed a benefit in overall survival after ablative treatment of up to 5 metastases (5). The most common primary tumor types in the study were breast (n = 18), lung (n = 18), colorectal (n = 18), and prostate (n = 16). For some of these tumor entities, such as lung and prostate cancer, specific randomized trials have been published, and they confirmed an oncologic benefit of metastasis-directed radiotherapy (MDRT) (2,6–8). Additionally, there is growing evidence that MDRT may improve outcomes and delay the need for a change in systemic therapy in oligorecurrent and oligopersistent OMD patients (9).
In breast cancer, up to 20% of patients with metastatic disease present with OMD (10). Nevertheless, the evidence regarding the benefit of MDRT in these patients is sparse (11). Data from the randomized controlled trial of standard-of-care systemic therapy with or without stereotactic body radiotherapy or surgical resection for newly oligometastatic breast cancer (NRG-BR002) suggest that local ablative therapy in addition to systemic therapy does not improve progression-free or overall survival in de novo breast cancer with OMD (1–4 metastases) (12). The CURB trial, on the other hand, investigates the benefit of MDRT in oligoprogressive breast and lung cancer patients. In a preplanned interim analysis, the authors showed a progression-free survival benefit. However, the benefit was driven only by the non–small cell lung cancer patients, and no benefit was observed for breast cancer patients (9). Even though the results of other randomized trials are pending, the recent data raise questions regarding the role of MDRT in OMD in breast cancer. The value of MDRT in breast cancer is of particular interest because of the large patient collective with OMD and favorable characteristics (luminal A/B, solitary metastasis, bone-only metastasis, long metastasis-free interval), which are associated with a chance of long-term survival (11).
So far, the definition of OMD depends solely on the number of imaging-detected metastases, and no clinical or molecular biomarkers exist to aid the detection of OMD (13–17). The European Society for Medical Oncology guidelines of 2021 define OMD as a maximum of 5 metastatic lesions, all susceptible to ablative local treatment (18). Thus, given the large differences in sensitivity, the imaging modality has an important impact on the OMD definition (14). Previous studies demonstrated that 18F-FDG PET/CT had a significantly higher sensitivity in detecting breast cancer metastases than did conventional imaging (19). Furthermore, 18F-FDG PET/CT allows differentiation of active from inactive metastases. Nevertheless, to our knowledge, the impact of 18F-FDG PET/CT on the definition of breast cancer with OMD has not been investigated. This is of importance, since unlike tumor entities such as lung cancer and prostate cancer, 18F-FDG PET/CT is not considered a standard procedure for staging in high-risk breast cancer patients (20).
MATERIALS AND METHODS
Patient Collective
The retrospective analysis was approved by the local institutional review board (2022-432-S-NP), and the requirement to obtain informed consent was waived. All patients in the current study underwent 18F-FDG PET/CT for breast cancer between October 2006 and January 2022 at the department of nuclear medicine at the Technical University of Munich.
The study included patients with oligometastatic breast cancer, defined as 1–5 distant metastases. Patients with brain metastases or a history of a second malignancy were excluded from the study. In total, the study enrolled 175 patients with 345 18F-FDG PET/CT scans obtained at different times during the course of their disease. After exclusion of scans that showed no change in metastatic pattern from the previous scan, 80 patients with 150 18F-FDG PET/CT scans remained for analysis.
The consensus recommendation of the European Society for Radiotherapy and Oncology and the European Organization for Research and Treatment of Cancer (21) was used to characterize OMD as follows: de novo OMD: first time diagnosis of metastatic disease, repeat OMD: previous history of OMD, and induced OMD: previous history of polymetastatic disease.
Image Acquisition
18F-FDG PET/CT scans were acquired using a Siemens Biograph 64 (2006–2011; n = 6) or a Siemens mCT128 (2012–2022; n = 144). The amount of activity injected was adjusted per body weight as recommended by the European Association of Nuclear Medicine guidelines (22). Patients with hyperglycemia were excluded from the study. The CT images of the 18F-FDG PET/CT scans were of diagnostic quality. Oral contrast medium was administered to all patients. Patients also received intravenous contrast medium unless there were contraindications, such as significantly impaired renal function or previous allergic reactions. For the diagnostic CT scan, imaging was started in the portal venous phase with the arms raised above the head unless the patient was unable to do so for the duration of the 18F-FDG PET/CT scan. The tube current was modulated according to an exposure control scout scan. Axial CT images of 3- or 5-mm thickness were reconstructed and displayed together with the corresponding 18F-FDG PET/CT images on a Siemens Syngo. In addition, a breath-hold CT scan was acquired for improved detection of small pulmonary nodules.
Assessment of Metastatic Pattern
Each 18F-FDG PET/CT image underwent evaluation by an experienced specialist in nuclear medicine and radiology. For each case, the interdisciplinary team analyzed first conventional CT images alone (conventional CT) and subsequently 18F-FDG PET/CT images. The images, clinical information, and corresponding clinical reports (of only the corresponding imaging modality) were available for the assessment of metastases. For each metastasis, we recorded the localization and assessed the congruence between conventional CT and 18F-FDG PET/CT (Fig. 1). Data were analyzed using SPSS version 26.0.
Comparison of conventional CT and 18F-FDG PET/CT. (A) Congruence between CT and 18F-FDG PET/CT: suspected metastasis on CT with confirmation on 18F-FDG PET/CT. (B and C) No congruence between imaging modalities: suspected metastasis on CT rated as nonspecific lesion on 18F-FDG PET/CT (B) and nonspecific finding on CT images (occult metastases) with suggestive presentation on 18F-FDG PET/CT (C).
RESULTS
Patient Collective
The patients’ characteristics can be found in Table 1. Thirty-two (21.3%) cases were classified as de novo OMD (group 1 according to the consensus recommendation of the European Society for Radiotherapy and Oncology and the European Organization for Research and Treatment of Cancer), 61 cases as repeat OMD (group 2), and 57 cases as induced OMD (group 3). A detailed description is provided in Table 2. Thirty-two patients (21.3%) had no systemic therapy for metastatic disease before the staging, and in 118 cases (78.7%), patients had systemic therapy for metastatic disease before 18F-FDG PET/CT.
Patient Characteristics
Classification of OMD According to Consensus Recommendation of European Society for Radiotherapy and Oncology and European Organization for Research and Treatment of Cancer
Differences Between 18F-FDG PET/CT and Conventional CT
In only 54.7% (n = 82) of cases was OMD diagnosed on both imaging modalities. In the remaining cases, either polymetastatic disease or no distant metastases were detected on 1 of the 2 imaging modalities (Table 3).
Number of Cases with OMD on 18F-FDG PET/CT Only, on Conventional CT Only, or on Both Imaging Modalities
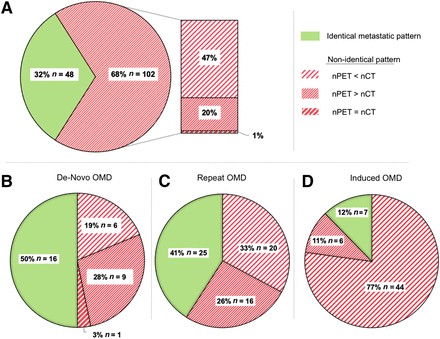
The proportion of patients with an identical metastatic pattern on conventional CT and 18F-FDG PET/CT was low, at 32% (n = 48) (Fig. 2). In most cases, the number of metastases (n = 54; 36%), the location of metastases (n = 2; 1.3%), or both (n = 46; 30.7%) differed between18F-FDG PET/CT and conventional CT.
Comparison of metastatic pattern between conventional CT and 18F-FDG PET/CT. (A) All groups in European Society for Radiotherapy and Oncology and European Organization for Research and Treatment of Cancer (ESTRO/EORTC) consensus recommendations. (B–D) Collective differentiated by ESTRO/EORTC OMD classification: ESTRO/EORTC group 1: de novo OMD (B); ESTRO/EORTC group 2: repeat OMD (C); and ESTRO/EORTC group 3: induced OMD (D).
In patients with de novo OMD, we observed the best congruence between 18F-FDG PET/CT and conventional CT, whereas in patients with induced OMD, only 12.3% of cases had an identical metastatic pattern on both imaging entities. The differences were attributed mostly to suggestive findings on conventional CT that were rated as unspecific lesions or inactive metastases on 18F-FDG PET/CT (Fig. 3).
Congruence of 18F-FDG PET/CT findings with conventional CT. (A) 18F-FDG PET/CT–positive metastases that were detected on conventional CT (green bars). (B) Difference in detected metastases between imaging modalities (y) and percentage of 18F-FDG PET/CT metastases that were also detected on conventional CT (x). (C) Number of 18F-FDG PET/CT–positive metastases (y) compared with number of PET-positive metastases that were also detected on conventional CT (x). Cases with polymetastatic disease on either conventional CT or 18F-FDG PET/CT are excluded from this analysis. Green circles = cases with congruent findings on conventional CT and 18F-FDG PET/CT. Red circles = cases with noncongruent finding in ≥1 metastatic lesion. Diameter of circles correlates with number of cases (small numbers to right).
In 50 cases (33.3%), 18F-FDG PET/CT revealed metastases that were not detected with conventional CT. In 31 (20.7%) cases, less than 50% of 18F-FDG PET/CT–positive lesions were detected on conventional CT (Fig. 3).
Most metastases in our study were in the skeletal system (49.5%), followed by the nonregional lymph nodes (27.1%). The largest deviation between conventional CT and 18F-FDG PET/CT was in the skeletal system. Here, the differences were attributed mostly to pathologic findings on conventional CT that were rated as unspecific lesions or inactive metastases on 18F-FDG PET/CT.
In the nonregional lymph system, on the other hand, 24.3% of metastases were detected only on 18F-FDG PET/CT, being occult on conventional CT. The results are summarized in Table 4.
Congruence of Metastases Between Conventional CT and 18F-FDG PET/CT in Different Organ Systems
DISCUSSION
Both the definition and the optimal treatment of OMD are subjects of current research. Currently, the definition of OMD is based on the imaging-detected number of metastases. A 2020 consensus report by the European Society for Radiotherapy and Oncology and American Society for Radiation Oncology defined OMD as a disease with 1–5 metastatic lesions that must be safely treatable regardless of the status of the primaries (23). In this report, the authors emphasized the importance of diagnostic imaging: 92% of participants agreed that there are minimum imaging requirements to define an oligometastatic state and that “diagnostic imaging should be performed using whichever modalities are adequate to image sites of common metastases and to detect small lesions for that histology” (23). Eighty-two percent of participants agreed that 18F-FDG PET/CT should be performed. Nevertheless, to our knowledge, in none of the large ongoing randomized trials investigating the role of MDRT in breast cancer, 18F-FDG PET/CT staging is mandatory.
The large differences between 18F-FDG PET/CT and conventional CT in our study were attributed to either additional metastases on 18F-FDG PET/CT compared with conventional CT (scenario 1) or metastatic lesions on conventional CT without pathologic 18F-FDG uptake rated as inactive metastases or an unspecific finding (scenario 2).
Both scenarios have important clinical implications. Scenario 1 applied to a third of our study collective. Differences were particularly pronounced for nonregional lymph nodes (Table 4), as can be explained by the low sensitivity (46%) of CT for lymph node metastases (24). This low sensitivity for lymph node metastases can be attributed to the fact that distinction is based solely on the size and shape of the lymph node. 18F-FDG PET/CT, on the other hand, provides additional information about metabolic activity, resulting in a better sensitivity for detecting distant metastases. Niikura et al. compared 18F-FDG PET/CT data with findings on biopsy, subsequent imaging, or clinical follow-up in 225 breast cancer patients (25). The sensitivity and specificity in the detection of distant metastases were 97.4% and 91.2%, respectively, for 18F-FDG PET/CT and 85.9% and 67.3%, respectively, for conventional imaging (CT, ultrasonography, radiography, and skeletal scintigraphy). Further studies on 18F-FDG PET/CT in breast cancer report even higher sensitivity and specificity (>98%) for the detection of distant metastases (26–28). The false-positive rate for the detection of metastatic disease is reported to be low, with values below 5% (27). Groheux et al. observed that 18F-FDG PET/CT detected unsuspected metastases in more than 45% of patients with locally advanced breast cancer (29). Compared with this, the number of additional metastases was slightly lower in the current study.
A high sensitivity for the detection of metastases is crucial for the concept of ablative treatment in OMD, which requires that all active lesions be treated with curative intent to erase the potential sites of origin for further metastasis or local progression (30,31). The persistence of a single unrecognized metastasis can jeopardize the success of ablative therapy in OMD (32): the ORIOLE trial randomized prostate cancer patients with OMD in stereotactic body radiotherapy versus observation alone. The treatment plan was based on conventional imaging alone, although prostate-specific membrane antigen PET/CT was available. At baseline, in 44%, prostate-specific membrane antigen PET/CT revealed additional positive lesions compared with conventional imaging. A post hoc analysis showed that the extent of undetected metastases on conventional imaging had a direct impact on the oncologic outcome (8,33): total consolidation of prostate-specific membrane antigen radiotracer–avid disease decreased the risk of new lesions at 6 mo (16% vs. 63%; P = 0.006) (8). In the controlled trial of standard-of-care systemic therapy with or without stereotactic body radiotherapy or surgical resection for newly oligometastatic breast cancer (NRG-BR002), new metastases outside index areas as first failure occurred in 40% of cases both in the treatment arm (receiving stereotactic ablative body irradiation and systemic therapy) and in the control arm (systemic therapy). 18F-FDG PET/CT staging was not mandatory. Given the large number of patients with additional metastases on 18F-FDG PET/CT in our study, it can be hypothesized that 18F-FDG PET/CT–directed MDRT could lead to a better oncologic outcome. This is reinforced by the excellent outcomes of small single-arm studies that investigated 18F-FDG PET/CT–directed MDRT in OMD in breast cancer, with local control of 95% and overall survival of 95% (34,35). Nonetheless, data directly evaluating the value of 18F-FDG PET/CT in OMD in breast cancer are missing.
Scenario 2 in which 18F-FDG PET/CT revealed fewer metastases than conventional CT applied to 46.7% of cases. In most of these cases, before staging, patients had systemic therapy that led to inactive metastases with normalized glucose metabolism on 18F-FDG PET/CT. In de novo OMD, we found a surprisingly high number of lesions that were positive only on conventional CT, compared with earlier studies (29,36). This can be explained by the fact that the previous studies evaluated 18F-FDG PET/CT for initial (preoperative) staging, whereas in our study, most patients in group 1 (de novo) had metachronous metastases and thus in most cases prior systemic adjuvant or neoadjuvant treatment. Overall, in our study only 12.3% of cases with induced OMD had the same metastatic pattern on conventional CT and 18F-FDG PET/CT. Thirty-six percent of cases with OMD were detected on only 18F-FDG PET/CT. Thus, use of only conventional CT is not suitable to reliably detect oligopersistent or oligorecurrent disease. In conventional CT, distinguishing between active metastases and inactive residual metastases is not feasible. However, bone scintigraphy in addition to conventional CT is likely to mitigate the large disparities observed in this study between 18F-FDG PET/CT and conventional CT.
Given the large number of patients with repeat and induced OMD that are OMD only on 18F-FDG PET/CT, 18F-FDG PET/CT (or at least conventional CT and bone scintigraphy) should be used as a reference for breast cancer staging and treatment monitoring when metastasis-directed therapy in these patients is being considered. Given the improvements in systemic therapies in recent years, the role of MDRT in repeat OMD or induced OMD is gaining importance. Ablative treatment of residual or progressive oligometastases allows the eradication of potential biologically altered metastases and, in some cases, the continuation of a well-tolerated and effective systemic therapy. Our results imply that the imaging modality in this patient cohort may play a particularly important role because the differences between conventional imaging and 18F-FDG PET/CT are here more pronounced than in the de novo situation.
Potential limitations of the current study include its retrospective design, potential interobserver variability, inclusion of heterogeneous groups of patients, and use of different 18F-FDG PET/CT systems. Additionally, 18F-FDG PET/CT was compared solely with its CT component, with no reference to histopathologic findings. Consequently, the study results are influenced by the sensitivity of imaging and the possibility of false-positive findings. It is crucial to note that the current study did not assess the accuracy of imaging modalities but rather highlighted differences in OMD definition attributable solely to the use of a different imaging modality.
Although both the sensitivity and the specificity of 18F-FDG PET/CT are reported to be high for detecting distant metastases in breast cancer patients (24–28,37), some molecular subtypes, such as lobular cancer, may benefit from 18F-flouroestradiol PET/CT for superior detection of estrogen receptor–positive distant metastases (38). Therefore, the use of more specific PET/CT tracers could potentially yield even larger differences than observed in the current study, which investigated exclusively 18F-FDG PET/CT. Despite its limitations, this study, to our knowledge, was the first to demonstrate the impact of 18F-FDG PET/CT on defining OMD in breast cancer. It underscores the need for a standardized definition of imaging modality when OMD is being characterized in breast cancer patients.
CONCLUSION
In breast cancer patients with OMD, 18F-FDG PET/CT leads in a third of cases to the detection of additional metastases. Furthermore, conventional CT alone is not suitable for reliably detecting patients with repeat or induced OMD. Thus, the use of 18F-FDG PET/CT has a substantial impact on the definition of OMD and detection of metastatic patterns in breast cancer patients. A standardized definition of the imaging modality is recommended for future trials of, and clinical practice regarding, metastasis-directed therapy in breast cancer patients.
DISCLOSURE
No potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: What is the impact of 18F-FDG PET/CT on detection and diagnosis of OMD in breast cancer?
PERTINENT FINDINGS: In almost half the cases, the imaging modality (18F-FDG PET/CT vs. conventional CT) had an impact on the assessment of whether OMD was present. An identical metastatic pattern was observed in only 32% of cases. 18F-FDG PET/CT detected additional metastases in 33.3% of cases.
IMPLICATIONS FOR PATIENT CARE: The imaging modality has a large impact on the definition of OMD in breast cancer, and conventional CT staging may not be the optimal for future trials regarding OMD in breast cancer.
Footnotes
Published online Apr. 18, 2024.
- © 2024 by the Society of Nuclear Medicine and Molecular Imaging.
REFERENCES
- Received for publication October 23, 2023.
- Revision received March 5, 2024.