Abstract
90Y-microsphere radioembolization has become a well-established treatment option for liver malignancies and is one of the first U.S. Food and Drug Administration–approved unsealed radionuclide brachytherapy devices to incorporate dosimetry-based treatment planning. Several different mathematical models are used to calculate the patient-specific prescribed activity of 90Y, namely, body surface area (SIR-Spheres only), MIRD single compartment, and MIRD dual compartment (partition). Under the auspices of the MIRDsoft initiative to develop community dosimetry software and tools, the body surface area, MIRD single-compartment, MIRD dual-compartment, and MIRD multicompartment models have been integrated into a MIRDy90 software worksheet. The worksheet was built in MS Excel to estimate and compare prescribed activities calculated via these respective models. The MIRDy90 software was validated against available tools for calculating 90Y prescribed activity. The results of MIRDy90 calculations were compared with those obtained from vendor and community-developed tools, and the calculations agreed well. The MIRDy90 worksheet was developed to provide a vetted tool to better evaluate patient-specific prescribed activities calculated via different models, as well as model influences with respect to varying input parameters. MIRDy90 allows users to interact and visualize the results of various parameter combinations. Variables, equations, and calculations are described in the MIRDy90 documentation and articulated in the MIRDy90 worksheet. The worksheet is distributed as a free tool to build expertise within the medical physics community and create a vetted standard for model and variable management.
- selective internal radiation therapy (SIRT)
- radioembolization
- partition model dosimetry
- 90Y-microspheres
- MIRD dual-compartment dosimetry
Selective internal radiation therapy (SIRT), or 90Y-microsphere radioembolization, is a therapy indicated for primary or metastatic hepatocellular carcinoma. Treatment entails use of a catheter positioned in a hepatic artery under fluoroscopic guidance to deliver glass or resin microspheres containing 90Y. The microspheres may become selectively entrapped within the tumor microvasculature to impart therapeutic radiation to the immediately adjacent tissue. Current SIRT protocols involve patient-specific prescribed activities—necessary because of variations in arterial vasculature and anatomy among different patients. Individualized treatment plans support optimization of therapeutic outcomes while minimizing radiation-induced side effects (1). Treatment planning is also part of the Food and Drug Administration–approved and Conformité Européenne–marked use of this modality. 99mTc-macroaggregated albumin (99mTc-MAA) is used as a surrogate agent for mapping the expected distribution of 90Y-microspheres before the therapeutic administration (2). The 99mTc-MAA imaging provides estimates of the 90Y-microsphere distribution via 2-dimensional planar γ-camera or 3-dimensional SPECT imaging—the latter preferred since planar imaging may overestimate the lung shunt fraction (LSF) (3,4). 99mTc-MAA images are used to evaluate extrahepatic shunting to the lung (5,6) to ensure that healthy lung tissue does not receive an absorbed dose greater than 30 Gy per treatment or a 50 Gy cumulative absorbed dose (for TheraSphere; Boston Scientific) (7) or to ensure that the procedure does not result in greater than a 20% lung shunt (for SIR-Spheres; Sirtex Medical Inc.) (8).
Historically, and currently, use of 90Y-microspheres has been approved with simple models for activity personalization, namely body surface area (BSA) and MIRD single compartment. Looking forward, increasingly customized dose models for greater levels of personalization in 90Y therapies are being introduced and evaluated. The 99mTc-MAA mapping images can be used in conjunction with various model-based dose calculation methods to determine a patient-specific administered activity for the 90Y-microsphere therapy. The recent standard operational procedures publication from the European Association of Nuclear Medicine dosimetry committee endorses the concept of integrating 99mTc-MAA mapping for therapy optimization (2), as does the International Commission on Radiation Units and Measurements (9) and other international scientific advisory bodies (10).
The BSA model for therapy planning was developed in the early 2000s (11) and is the established prescription activity model for the SIR-Spheres device (8). The BSA model has advantages of simplicity and ease of use (12). The BSA model assumes that the size of the patient’s total liver volume is proportional to the patient’s BSA, thereby enabling adjustment of prescribed activity to a patient’s malignant liver volume (13). Although the BSA approach has the advantage of simplicity (Fig. 1), the limited personalization may increase the risk of undertreatment or overtreatment of patients (14,15). One limitation of the BSA method is that it assumes the BSA correlates with liver volume in the general population; however, the validity of this assumption may vary depending on body habitus and disease burden (13). Consequently, the BSA model for 90Y treatment planning may lead to substantial interpatient variability in both the normal-liver–absorbed dose and the tumor-absorbed dose. For example, large patients with a disproportionately small liver volume may receive elevated absorbed doses to the nontumor regions of the liver, whereas smaller patients with larger livers may receive comparatively low absorbed doses when BSA-based planning is used (12,13). A second limitation is that neither tumor nor normal-liver parenchyma target absorbed doses are calculated or considered. Rather, the prescribed activity calculation formula is empiric, based on BSA, tumor volume, perfused liver volume, and whole liver volume. The calculation does allow for activity reduction of prescribed activity for clinical reasons or if the lung-absorbed dose, based on estimated LSF and lung mass, is estimated to exceed 30 Gy. Finally, the BSA model disregards the tumor–to–normal-liver activity concentration ratio (TNR). Nonetheless, the BSA model is still widely used to prescribe 90Y activity in the treatment of liver cancer, as this model remains the vendor-recommended dosimetry method in the United States for the SIR-Spheres device in the treatment of unresectable metastatic liver tumors (8). However, other factors such as tumor burden and liver function should also be considered to ensure optimal treatment outcomes (16).
Summary of various 90Y prescription activity models with associated compartments and assumed distribution. (A) Ground truth sphere distribution. (B) BSA model—considers volumes of 3 regions, including tumor, perfused liver, and whole liver volumes. (C) MIRD single compartment—considers only target region volume. (D) MIRD dual compartment—considers perfused normal-liver region volume and tumoral liver volume. (E) MIRD multicompartment—considers perfused normal-liver region volume, tumoral liver volume, and individual tumor volumes. (F) Voxel-level distribution.
The MIRD Committee of the Society of Nuclear Medicine and Molecular Imaging developed a standardized method for calculating absorbed radiation dose to macroscopic anatomic regions from β-particles emitted by radionuclides (17). This model, referred to in this paper as the MIRD single-compartment model (Fig. 1) for 90Y dosimetry, was predicated on the assumption that the distribution of 90Y within the liver can be approximated as a single region with a spatially uniform activity distribution and an absorbed fraction equal to 1.0 (17). This model suggests that a given activity concentration can be used to ascertain absorbed dose to the tissue by modeling the energy deposited per unit mass (absorbed dose, where 1 J/kg = 1 Gy). Thus, operators may calculate the prescribed activity required to deliver a certain absorbed dose (Gy) to a target tissue. This model uses volumetric measurements from anatomic imaging to determine the anticipated mass (volume × density) of distribution, from which the expected activity concentration (activity per unit mass) and absorbed dose may be derived. Vendor implementation of the single-compartment model incorporates uptake measurements from surrogate 99mTc-MAA imaging to account for anticipated microspheres shunted to the lungs. The MIRD single-compartment model is relatively simple, easy to implement, and more advanced than the BSA model because it uses a measurement of the mass of the patient-specific treatment region and allows for absorbed dose estimates to be integrated into the treatment procedure. However, its limitations include the inability to account for different distributions of 90Y within the healthy and diseased tissue in the treatment volume. This model is widely used today for 90Y dosimetry in liver cancer treatment and remains the approved method for TheraSphere treatment planning (7).
The partition model for 90Y SIRT treatment planning addresses the limitations of the BSA model and the MIRD single-compartment model (18). The partition model, here and in the MIRDy90 software, is called the MIRD dual-compartment model. The dual-compartment model accounts for differences in 90Y activity concentration within normal liver and subliver treatment regions to provide a more accurate method for computing absorbed dose in the various tissue compartments. Calculated absorbed doses may then be used to tailor the patient’s prescribed activity. The MIRD dual-compartment model defines the treatment volume as 2 compartments: the tumor region and the perfused liver region (Fig. 1D). In addition to using mass (volume × density) information for each region that is derived from volumetric anatomic imaging, the model also uses a TNR determined from the 99mTc-MAA SPECT images; from this information the absorbed dose in each region may be calculated (12). Thus, the model is more complex than the BSA and MIRD single-compartment models because it incorporates more patient-specific information, yet it still can be implemented with a small number of measurements and calculation parameters. The MIRD dual-compartment model can be used to deliver a threshold absorbed dose to tumor while maintaining safe absorbed doses to the liver and lungs. Limitations of the MIRD dual-compartment model are that it assumes uniform distribution of 90Y within the 2 regions (15) and cannot handle multiple tumor regions. To overcome these limitations, multicompartment models have been developed extending the dual-compartment model to accommodate individual tumors (19,20). Alternative voxel-based approaches have also been proposed for 90Y treatment planning (21–23), leading to the integration of voxel-based solutions by several vendors.
The BSA, single-compartment, and dual-compartment models rely on surrogate 99mTc-MAA imaging for estimating the lung-absorbed dose. The dual-compartment model relies on this surrogate for estimating the anticipated 90Y TNR. Riveira-Martin et al. showed that dual-compartment treatment planning using 99mTc-MAA resulted in projected absorbed dose metrics having a high correlation with posttherapy 90Y bremsstrahlung SPECT imaging, highlighting the utility of 99mTc-MAA surrogate imaging for 90Y treatment planning (24). Other studies reported important limitations when using 99mTc-MAA as a surrogate for 90Y-microspheres that influenced absorbed dose projections. For example, these limitations included different physical and biologic properties of 99mTc-MAA and microspheres (particle size and shape, and number of particles administered), catheter placement, and the different imaging modalities used for evaluation (e.g., partial-volume effects) (25–27). Despite the uncertainty regarding concordance between pretherapy 99mTc-MAA and 90Y-microsphere distributions, 99mTc-MAA imaging is readily available as a common diagnostic radiopharmaceutical, and its use is a required step in current treatment protocols.
There is significant emerging motivation for adopting more complex dosimetry models for 90Y SIRT treatment planning; several studies have suggested that the increased accuracy of the partition model may improve 90Y treatment planning compared with the simpler MIRD single-compartment and BSA models (18,27,28). For example, Garin et al. described a randomized multicenter study that assessed the benefit of personalized dosimetry (target tumor-absorbed dose with limited absorbed doses to the healthy liver and lungs) and standard dosimetry (target absorbed dose to the perfused lobe) in patients with hepatocellular carcinoma. The study of Garin et al. reported that personalized dosimetry in SIRT resulted in a significantly higher objective response rate than did the standard dosimetry approach, suggesting that personalized dosimetry could be beneficial in clinical practice and should be implemented in future clinical trials (29). In addition, recent recommendations from an international multidisciplinary workgroup explicitly recommend use of the dual-compartment model in certain clinical scenarios (10).
To the best of our knowledge, no MIRD dual-compartment model calculation tools are currently approved by the U.S. Food and Drug Administration. Approved voxel-level dosimetry software is available and can effectively provide dual-compartment dosimetry. Therefore, centers that do not use vendor software but wish to use the MIRD dual-compartment or multicompartment models are constrained to in-house calculation tools. There are several smartphone applications that have been available to prepare microsphere treatment plans. These apps have demonstrated the utility of having shared tools to support SIRT programs, where physicians, physicists, and others can review and compare dose calculations and prescribed activities. However, the apps provide black-box calculations, and neither the apps nor their function are well documented. Thus, the need remains for vetted MIRD dual-compartment and multicompartment model calculation tools.
In this article, we describe MIRDy90, a committee-tested and endorsed activity/absorbed dose calculation worksheet to support SIRT dosimetry calculations as one of the MIRDsoft.org suite of research dosimetry tools. The MIRDy90 worksheet has been tested and validated against other absorbed dose calculation tools.
MATERIALS AND METHODS
90Y-Microsphere Activity Calculation Models
The SIR-Spheres BSA model requires several input parameters: total treatment volume, tumor volume, LSF, patient height and weight, and total liver volume. As input for the BSA model, a BSA needs to be calculated. Although several published equations provide BSA, the equation used in the SIR-Spheres package insert is shown in Equation 1. The patient BSA is determined from the patient’s height and body mass: Eq. 1
Eq. 1
The BSA model-prescribed activity  can be calculated using Equation 2 with treatment, tumor, and total liver volumes obtained from the patient’s CT or MR images.
can be calculated using Equation 2 with treatment, tumor, and total liver volumes obtained from the patient’s CT or MR images. Eq. 2where V(tumor) is the volume of the tumor, V(PLV) is the volume of the perfused liver being treated, and V(total liver) is the volume of the whole liver. Along with the prescribed activity, the BSA model requires that the absorbed dose to the lung also be calculated and considered. This is achieved by first determining the zero-time activity within the lung (30):
Eq. 2where V(tumor) is the volume of the tumor, V(PLV) is the volume of the perfused liver being treated, and V(total liver) is the volume of the whole liver. Along with the prescribed activity, the BSA model requires that the absorbed dose to the lung also be calculated and considered. This is achieved by first determining the zero-time activity within the lung (30): Eq. 3
Eq. 3
The absorbed dose to the lung is calculated as follows: Eq. 4where M(lung) is the mass of the lungs, typically assumed to be 1 kg, and DCF is the 90Y dose conversion factor. The DCF relates the energy deposited locally in tissue per unit of activity, expressed in units of J/GBq. In the 90Y dosimetry models described herein, the DCF values commonly used assume charged particle equilibrium and complete self-absorption; this assumption results in a slight overestimate of the absorbed dose depending on the microsphere distribution volume (Fig. 4 in reference 2). Various publications provide different values for the DCF: American Association of Physicists in Medicine report 144 and International Commission on Radiation Units and Measurements report 96 use 49.38 J/GBq (9,31), the SIR-Spheres package insert uses 49.67 J/GBq (8), the TheraSphere treatment calculator uses 50 J/GBq (7), and the European Association of Nuclear Medicine dosimetry committee recommends a value of 49.75 J/GBq (2). Values of 49.67 J/GBq and 49.77 J/GBq can be derived from National Nuclear Data Center (32) and MIRD (33) decay data, respectively. The differences appear to be due to small variations in the source nuclear data, such as the average energy emitted per nuclear transition and isotope half-life. MIRDy90 is prepopulated with the MIRD decay data–derived DCF of 49.77 J/GBq.
Eq. 4where M(lung) is the mass of the lungs, typically assumed to be 1 kg, and DCF is the 90Y dose conversion factor. The DCF relates the energy deposited locally in tissue per unit of activity, expressed in units of J/GBq. In the 90Y dosimetry models described herein, the DCF values commonly used assume charged particle equilibrium and complete self-absorption; this assumption results in a slight overestimate of the absorbed dose depending on the microsphere distribution volume (Fig. 4 in reference 2). Various publications provide different values for the DCF: American Association of Physicists in Medicine report 144 and International Commission on Radiation Units and Measurements report 96 use 49.38 J/GBq (9,31), the SIR-Spheres package insert uses 49.67 J/GBq (8), the TheraSphere treatment calculator uses 50 J/GBq (7), and the European Association of Nuclear Medicine dosimetry committee recommends a value of 49.75 J/GBq (2). Values of 49.67 J/GBq and 49.77 J/GBq can be derived from National Nuclear Data Center (32) and MIRD (33) decay data, respectively. The differences appear to be due to small variations in the source nuclear data, such as the average energy emitted per nuclear transition and isotope half-life. MIRDy90 is prepopulated with the MIRD decay data–derived DCF of 49.77 J/GBq.
The MIRD single-compartment model assumes that the activity of 90Y is distributed uniformly throughout the target treatment site. The absorbed dose and prescribed activity are calculated using the activity of 90Y and physical half-life, dose deposition characteristics, and the volume of the liver region being treated. The model requires the target region (perfused liver/treatment volume) mass (mass = volume × density), the LSF, and the desired absorbed dose to the target region. The desired absorbed dose is defined in Equation 5: Eq. 5
Eq. 5
The prescribed activity is then calculated using Equation 6, which accounts for shunting of spheres by including a correction for the LSF: Eq. 6
Eq. 6
The MIRD dual-compartment model requires several patient-specific inputs: the desired absorbed dose to the tumoral liver compartment (Gy), target volume (the perfused normal liver volume plus tumor volume), tumor volume, LSF, and TNR. To calculate the 90Y prescribed activity, the MIRD dual-compartment model requires the volumes of the tumor and normal perfused liver in the lobe being treated and the anticipated uptake in the tumor, perfused normal liver, and lung, typically measured using the pretreatment 99mTc-MAA SPECT. The TNR is the ratio of activity concentration in the tumoral liver to that in the perfused normal liver tissue (Eq. 7). The TNR calculation is imperfect; segmented volumes of interest on SPECT/CT images can vary with segmentation technique and have inter- and intraoperator variability or can be subject to partial-volume effects (34). The TNR in Equation 7 is unitless: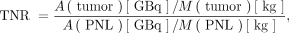 Eq. 7where A(tumor) is the activity in the tumoral liver, M(tumor) is the mass (kg) of the tumoral liver, A(PNL) is the activity in the perfused normal liver, and M(PNL) is the mass (kg) of the perfused normal liver. The absorbed dose to the tumor is
Eq. 7where A(tumor) is the activity in the tumoral liver, M(tumor) is the mass (kg) of the tumoral liver, A(PNL) is the activity in the perfused normal liver, and M(PNL) is the mass (kg) of the perfused normal liver. The absorbed dose to the tumor is Eq. 8where D(PNL) is the mean absorbed dose to the perfused normal liver tissue. The prescribed activity of 90Y is calculated as follows:
Eq. 8where D(PNL) is the mean absorbed dose to the perfused normal liver tissue. The prescribed activity of 90Y is calculated as follows: Eq. 9where A0,DC is the prescribed activity of microspheres calculated using the MIRD dual-compartment model. Using Equations 8 and 9, one can tailor prescribed activity to deliver a target absorbed dose to the tumoral liver, normal liver, or lung tissue.
Eq. 9where A0,DC is the prescribed activity of microspheres calculated using the MIRD dual-compartment model. Using Equations 8 and 9, one can tailor prescribed activity to deliver a target absorbed dose to the tumoral liver, normal liver, or lung tissue.
The MIRD dual-compartment model can be extended to a multicompartment tumor model by considering individual tumors that make up the tumoral liver compartment as separate compartments: Eq. 10where Di is the absorbed dose to the individual tumor (tumori), which is scaled according to the tumor TNR (TNRi) relative to the absorbed dose to the perfused normal liver. For the model to be implemented as presented in Equation 10, the absorbed dose to the perfused normal liver, D(PNL), needs to be calculated a priori. In MIRDy90, D(PNL) is calculated by implementing the dual-compartment model and must be determined before multicompartment calculations.
Eq. 10where Di is the absorbed dose to the individual tumor (tumori), which is scaled according to the tumor TNR (TNRi) relative to the absorbed dose to the perfused normal liver. For the model to be implemented as presented in Equation 10, the absorbed dose to the perfused normal liver, D(PNL), needs to be calculated a priori. In MIRDy90, D(PNL) is calculated by implementing the dual-compartment model and must be determined before multicompartment calculations.
MIRDy90 Calculation Worksheet
The MIRDy90 calculation worksheet was built as a tool for evaluating 90Y SIRT prescribed activity calculation models. Features of MIRDy90 include parallel implementation of BSA, MIRD single-compartment, MIRD dual-compartment, and MIRD multicompartment tumor model dosimetry; a simple-to-use single-screen interface; instant calculations for an interactive user experience, provided with supporting graphics; informative model summaries; straightforward and simple comparisons of activity calculation models; and easy-to-review (open-source) code and calculations.
The MIRDy90 worksheet was built on the Excel (Microsoft) platform, which allows an organized input–output user interface and reviewable calculations. The worksheet has been designed to be straightforward and uses the formatting and graphical capabilities of Excel. A screenshot of the MIRDy90 interface is shown in Figure 2.
Screenshot of MIRDy90 software graphical user interface. User input area is on left, and worksheet calculations are on right. In this example, MIRD dual-compartment model is selected, and target absorbed dose is prescribed to achieve 205 Gy to tumor. (A) Input data fields for each model. Input parameters used in selected model are highlighted. (B) Model summary box, which displays user’s selected calculation outputs. (C) Selected model graphical display and dose summary table. (D) MIRD dual-compartment dosimetry model outputting display activity and absorbed dose summaries for each of 4 prescribed activity targets. (E) MIRD multicompartment input (TNRi) and projected tumor-absorbed doses (for up to 5 tumors) (optional). (F) Single-compartment (MIRD) and BSA dosimetry model outputs providing conventional model summaries. DL = dose limit; PNL = perfused normal liver.
Workflow and Model Selection
Users may enter data based on simulation or case measurements, review and compare model estimates, select the desired model, and save the case or print the screen. The requisite parameters to support each model are displayed on the input screen. As users enter data into the fields, the available models become activated, and respective calculations are displayed. The user may navigate and select one of the models from the drop-down menu, as shown in Figure 3. The prescribed activity for the selected model is shown in the model summary box on the bottom left of the interface (Fig. 2B).
MIRDy90—selecting prescribed activity calculation model.
Once a model is selected, the requisite inputs required for the calculations are highlighted, along with the respective output dosimetry module. The dose summary table and graphical display (Fig. 2C) will show a summary of the prescribed activities and compartment doses, as well as indicators confirming whether the treatment exceeds any of the specified dose limits relevant to the selected model. The plot presents an overview of prescribed activity (GBq) versus absorbed dose (Gy), as well as relative doses in each of the compartments in the respective models.
MIRD Single-Compartment and BSA Models
If the MIRD single-compartment or BSA model is selected, the respective dosimetry module is highlighted (Fig. 2F). The dose summary table and graphical display (Fig. 2C) will show the conventional outputs for the single-compartment and BSA models. The MIRDy90 BSA model presents outputs akin to the SIR-Spheres 90Y Resin Microspheres Activity Calculator (SMAC; Sirtex Medical Inc.), a dose prescription calculator available online (35). The BSA model has the option for BSA activity reduction (%) or for dosing to the lung limit by selecting the model “BSA dose to lung limit.” The single-compartment dosimetry module summarizes the conventional single-compartment (MIRD) model inputs and outputs akin to the TheraSphere calculator (36).
MIRD Dual- and Multicompartment Models
If the MIRD dual-compartment model is selected, the MIRD dual-compartment dosimetry module (Fig. 2D) will be highlighted. The dose summary Table (Fig. 2C) and MIRD dual-compartment dosimetry module will display a summary of the prescribed activities and compartment doses, as well as indicators confirming whether treatment exceeds any of the specified liver-, lung-, and cumulative lung-absorbed dose limits. Users can refine calculations by adjusting parameters, aided by graphical display of the output absorbed doses. Users can select 1 of 4 target constraints: prescribed target absorbed dose to the tumor (Gy), absorbed dose limit to the liver, absorbed dose limit to the lung, or user-defined administered activity (GBq). In each scenario, the worksheet calculates the required prescribed activity. The displayed plot presents prescribed activity (GBq) versus dose (Gy), as well as the relative doses in each of the compartments. The plot updates automatically, depending on which of the 4 MIRD dual-compartment model targets is selected. Additionally, MIRDy90 facilitates calculation of individual tumor-absorbed doses in the multicompartment module, where users can provide individual tumor TNRs. In MIRDy90, individual tumor doses are scaled relative to the perfused normal-liver–absorbed dose according to Equation 10. If an individual tumor TNR is entered into the multicompartment table (Fig. 2E), an additional target constraint will be available in the prescribed activity calculation model drop-down list (Fig. 3). This model, “multicompartment (min target tumor dose),” calculates the prescribed activity required to deliver a minimum tumor-absorbed dose. In Figure 2, the dual-compartment model (target tumor dose) is selected, and the graphical display shows the prescribed activity of 90Y (dashed red line), as well as the absorbed doses to the tumor (dark blue line), perfused normal liver tissue (light blue line), and lung (green line). The dual-compartment dosimetry module also displays the prescribed activity and the compartment absorbed doses and activities for all target constraints simultaneously, highlighting only the model that is selected.
Default Parameters
MIRDy90 is prepopulated with default parameters required for the program to run, derived from reference publications. These include a liver tissue density of 1.03 g/cm3 (7), a DCF of 49.77 J/GBq (33), a default lung volume of 5,525 cm3 (equivalent to a lung mass of 1.0 kg based on an assumed mean lung density of ρ = 0.181 g/cm3 (37)), a perfused normal-liver dose limit of 70 Gy (38), a single-treatment lung dose limit of 30 Gy (7,8), and a cumulative lung dose limit of 50 Gy (7). The user may choose to modify these default parameters.
Benchmarking MIRDy90 Against Vendor Dose Prescription Tools
The MIRDy90 worksheet was designed to use the same equations as those provided with various vendor dosimetry tools. For the BSA model validation, the MIRDy90 worksheet results were compared with those from SMAC (35). The SMAC tool uses the BSA model to calculate the suggested prescribed activity for use of the product and estimates the lung-absorbed dose based on either the default 1-kg or entered patient-specific lung mass.
For the MIRD single-compartment model comparison, the MIRDy90 results were compared with those of the Boston Scientific iDOC online calculator used to determine TheraSphere prescribed activities. The iDOC tool (36) provides the TheraSphere 90Y-microsphere prescribed activity for treatment planning as well as the projected lung-absorbed dose; TheraSphere has recently removed this tool from its website indefinitely. For the dual-compartment model, benchmarking MIRDy90 results against a vendor-provided standard was not possible because no vendors currently have software for this region-based model. For external comparison of the dual-compartment model, MIRDy90 calculations were benchmarked against the Cancer Y90 Calculator (Softmills Technology International), a smartphone app currently available for android and iOS that provides single-compartment and dual-compartment model dosimetry (39).
A series of 10 example patient cases was generated by selecting typical clinical values determined from local experience. The example data were entered into the MIRDy90 worksheet, and the results of the various calculation models were compared with the respective available online calculators or app. The target volumes (perfused normal liver + tumoral liver) ranged from 400 to 850 cm3; tumor volumes, from 30 to 330 cm3; TNR values, from 2.8 to 5.4; LSF values, from 1% to 10%; patient heights, from 150 to 186 cm; patient weights, from 55 to 118 kg; and total liver volumes, from 1,000 to 1,603 cm3. The MIRD single-compartment target dose to the treatment volume was chosen to be 150 Gy, and the dual-compartment model desired absorbed dose to tumor was chosen to be 205 Gy. Each tool uses similar but slightly different DCFs, and the MIRDy90 tool allows adjustment of this factor by the user. For comparative purposes, the DCF used in MIRDy90 was adjusted to match the value used in the software tool to which it was compared (e.g., the SIR-Spheres SMAC and Cancer Y90 Calculator [partition] app use a DCF of 49.67 J/GBq, and the TheraSphere iDOC uses a DCF of 50 J/GBq).
The assumed soft-tissue density (tumoral and nontumoral liver) also varies among the tools, and this was adjusted in MIRDy90 to facilitate direct comparisons. For reference, the vendor-supplied TheraSphere instructions for use adopts a soft-tissue density of 1.03 g/cm3, and International Commission on Radiological Protection (ICRP) publications 110 and 145 use densities of 1.05 and 1.06 g/cm3, respectively (ICRP 110 considers only the parenchyma, whereas ICRP 145 considers contributions from both blood and parenchyma (40)); the Cancer Y90 Calculator app uses the ICRP 145 soft-tissue density of 1.06 g/cm3. Alternatively, lung density may be obtained directly from patient CT. The parameters for each of the simulated cases are shown in Table 1.
Simulated Patient Input Parameters
RESULTS
In comparing MIRDy90 calculations with those provided by vendors and external calculators, we found the results to be virtually identical; the variability observed resulted from different presentations of significant figures in the programs. The external calculators present calculations with 2 decimal places, whereas MIRDy90 uses 3 significant figures. A summary of the results is shown in Tables 2 and 3, and the ratios of values between MIRDy90 and the vendor tool or app calculators are shown in Table 4.
Prescription Activity Output from MIRDy90 and Vendor or App Calculators
Estimated Model Lung Absorbed Dose Output from MIRDy90 and Vendor or App Calculators
Ratio of Activity and Lung-Absorbed Dose Outputs Between MIRDy90 and Vendor or App Calculators
DISCUSSION
90Y-microsphere therapy protocols integrate 99mTc-MAA imaging to prospectively estimate the distribution 90Y-microspheres (31). Although research has shown that distributions of 99mTc-MAA and 90Y-microspheres are not necessarily concordant, pretreatment 99mTc-MAA imaging provides a first-order approximation of the retention of microspheres in tumor and healthy perfused liver tissues. We maintain that 90Y SIRT treatments can be improved with better planning, and the emerging method of choice to support this is the dual-compartment model (10,12,14,15,18,39). As the field evolves to more complex dosimetry, there are no free, vetted tools that can be used to help validate calculations. Therefore, we have developed a needed, tested, and validated standardized tool that can perform dual- and multicompartment model calculations transparently and interactively.
The MIRDy90 calculation worksheet was designed to educate and to facilitate research involving the calculation of 90Y SIRT prescribed-activity models. The MIRDy90 tool incorporates features such as parallel BSA, MIRD single-compartment, MIRD dual-compartment, and MIRD multicompartment model dosimetry calculations and offers a simple-to-use interface on a single screen, allowing for instant calculations accompanied by informative graphical visualization of the dosimetry outputs. The tool also provides helpful model summaries, enabling review and comparison of prescribed activities and absorbed doses among the various calculation models. The ability to determine dual-compartment model 90Y activity for 1 of 4 user-defined targets or to extend the calculations to individual tumor targets, and the ability to quickly switch among these options for calculations, provides enhanced user flexibility and control for determining prescribed activity. MIRDy90 may be used as a benchmarking or teaching tool or to facilitate quality assurance. Calculations in MIRDy90 with 10 simulated test cases using the BSA, MIRD single-compartment, and MIRD dual-compartment models closely matched the prescribed-activity and lung dose estimates obtained when the 3 validation software tools were used.
MIRDy90 does have limitations. The spreadsheet implements calculations based on the model assumptions stated above; it does not support voxel-level dosimetry or 3-dimensional visualization; it has not been reviewed or approved by regulatory agencies. Furthermore, like many equivalent dosimetry software solutions, the software outputs are dependent on user inputs, and use of the software requires understanding of both the input variables and the dose estimation models used.
CONCLUSION
The MIRDy90 worksheet was built on the Excel platform to enhance user accessibility and computations incorporating a familiar, single-screen graphical user interface, with input parameters and output results fully transparent to the user. The spreadsheet incorporates multiple models, according to user preference, and may be executed with many optional parameters for case-specific calculations. The spreadsheet was developed and tested by a competent team of medical physicists in collaboration with the full MIRD committee. Through intercomparison testing, we confirmed that the MIRDy90 worksheet provides reliable calculations needed to evaluate and support the use of 90Y-microspheres. The results of calculations compared favorably with other dosimetry tools. MIRDy90 is freely available to the nuclear medicine community as one element of the MIRDsoft.org community-tools initiative.
DISCLOSURE
This research was funded in part through the NIH/NCI Cancer Center support grant P30 CA008748 and NIH U01 EB028234. Joseph Rajendran of the MIRD committee is currently on the staff of the U.S. Food and Drug Administration. His contribution to this article as a reviewer was in his personal capacity. The views expressed are his own and do not represent the views of the Food and Drug Administration or the U.S. government. No other potential conflict of interest relevant to this article was reported.
ACKNOWLEDGMENTS
We thank Dr. Kathy Willowson and Prof. Dale Bailey for their insights and feedback throughout the development of MIRDy90. The members of the MIRD committee are Vikram Adhikarla, Rachel M. Barbee, Wesley E. Bolch, Yuni K. Dewaraja, William D. Erwin, Valentina Ferri, Darrell R. Fisher, Robert F. Hobbs, Roger W. Howell, Oleksandra V. Ivashchenko, Adam L. Kesner, Richard Laforest, Joseph G. Rajendran, George Sgouros, Carlos F. Uribe, and Pat B. Zanzonico (Chair). The MIRDy90 software aids a user in performing 90Y SIRT treatment planning calculations and is intended for educational and research use only. MIRDy90 has not been approved by the U.S. Food and Drug Administration and is not intended for clinical use or use as a medical device. MIRDy90 and any results generated from its use are not substitutes for medical diagnosis, advice, or treatment of specific medical conditions. A physician should always be consulted for any health problem or medical condition.
Footnotes
Published online Feb. 22, 2024.
- © 2024 by the Society of Nuclear Medicine and Molecular Imaging.
REFERENCES
- Received for publication October 3, 2023.
- Accepted for publication January 23, 2024.










