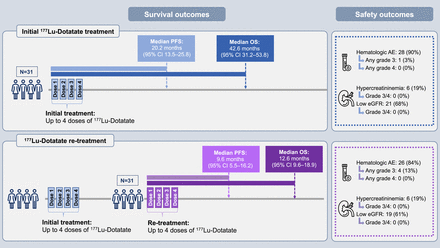
Visual Abstract
Abstract
Advanced neuroendocrine tumors (NETs) are associated with a poor prognosis. A regimen of 4 cycles of 177Lu-DOTATATE has been shown to improve both progression-free survival (PFS) and overall survival (OS) in patients with advanced NETs. To the best of our knowledge, this is the first study in the United States to evaluate the effectiveness and safety of additional cycles of 177Lu-DOTATATE therapy in patients with progressive NETs. Methods: This was a retrospective chart review of adults with advanced NETs. The patients had undergone initial treatment with up to 4 cycles of 177Lu-DOTATATE and, after disease progression and a period of at least 6 mo since the end of the initial treatment, were retreated with at least 1 additional cycle at a single center (2010–2020). Patient characteristics, treatment patterns, and clinical outcomes were evaluated descriptively. Response was evaluated according to RECIST 1.1; toxicity was defined using criteria from Common Terminology Criteria for Adverse Events, version 5.0. Kaplan–Meier plots were used to evaluate PFS and OS. Results: Of the 31 patients who received 177Lu-DOTATATE retreatment, 61% were male and 94% were White. Overall, patients received a median of 6 cycles (4 initial cycles and 2 retreatment cycles), and the mean administered activity was 41.9 GBq. Two patients also went on to receive additional retreatment (1 and 2 cycles, individually) after a second period of at least 6 mo and progression after retreatment. Best responses of partial response and stable disease were observed in 35% and 65% of patients after the initial treatment and 23% and 45% of patients after retreatment, respectively. The median PFS after the initial treatment was 20.2 mo and after retreatment was 9.6 mo. The median OS after the initial treatment was 42.6 mo and after retreatment was 12.6 mo. Hematologic parameters decreased significantly during both the initial treatment and retreatment but recovered such that there was little difference between the values before the initial treatment and before the retreatment. Clinically significant hematotoxicity occurred in 1 and 3 patients after the initial treatment and retreatment, respectively. No grade 3 or 4 nephrotoxicity was observed. Conclusion: Retreatment with 177Lu-DOTATATE after progression appeared to be well tolerated and offered disease control in patients with progressive NETs after initial 177Lu-DOTATATE treatment.
- advanced neuroendocrine tumors
- retreatment
- real-world outcomes
- 177Lu-DOTATATE
- peptide receptor radionuclide therapy
Neuroendocrine tumors (NETs) are a heterogeneous group of malignancies, most commonly originating in the gastrointestinal system, with varying proliferation rates and outcomes (1). The overall incidence of NETs has increased dramatically in the United States (U.S.) over the past 40 y, in part because of increasing awareness and improved diagnostic testing (2,3). For patients with localized NETs, surgical resection remains the only curative option. Recent studies have, however, demonstrated that 40%–50% of NETs are metastatic at diagnosis, which is associated with a poor prognosis and often requires more complex clinical management (1,4–8).
Current treatment options for patients with advanced NETs include locoregional therapies (e.g., radioembolization) and systemic therapies (e.g., somatostatin analogs, interferon-α, targeted therapies, cytotoxic chemotherapies, and peptide receptor radionuclide therapies) (9–19). Treatments are individualized according to the tumor type, the extent of the disease, and the level of symptoms and aim to prolong survival, to improve and maintain quality of life, and to control tumor growth and secretory symptoms (8,20). Despite the availability of an increasing number of systemic treatments, the prognosis for patients with advanced NETs remains poor, with the median overall survival (OS) ranging from only 4 mo to 6 y (20–22).
In January 2018, after the results of the phase 3 NETTER-1 trial were released (19,23), 177Lu-DOTATATE, a peptide receptor radionuclide therapy, was approved in the U.S. for the treatment of advanced NETs (24). In the NETTER-1 trial, treatment with 177Lu-DOTATATE plus a long-acting repeatable somatostatin analog (30 mg of octreotide) improved progression-free survival (PFS) and OS compared with 60 mg of high-dose, long-acting repeatable octreotide alone. The approved regimen activity is 7.4 GBq of 177Lu-DOTATATE given 8 wk apart for 4 cycles. Recent studies have demonstrated the efficacy of 177Lu-DOTATATE in patients with NETs (25); however, as most advanced NETs are incurable, patients will eventually progress, and when this occurs, the treatment options are limited. This has led to an interest in the possibility of retreating patients with 177Lu-DOTATATE on progression.
To date, there are few published data on the safety or efficacy of retreatment with 177Lu-DOTATATE, particularly from a U.S. perspective. Though a small number of prior studies have indicated that additional cycles of 177Lu-DOTATATE in the salvage setting are feasible, safe, and effective, these studies are mostly from European centers (21,23,26–28). The objective of the current study was to evaluate the real-world effectiveness and safety of retreatment with 177Lu-DOTATATE in patients with progressive NETs in the U.S.
MATERIALS AND METHODS
Study Design and Patient Selection
This was a retrospective review of patient medical records at a single U.S. center—the Excel Diagnostics and Nuclear Oncology Center in Houston, Texas. Adult (>18 y) patients with a diagnosis of a NET who received retreatment with 177Lu-DOTATATE between July 1, 2010, and December 31, 2020, were included. Included patients were required to have undergone initial treatment with up to 4 cycles of 177Lu-DOTATATE and, after disease progression and a period of at least 6 mo since the end of the initial treatment, had to have received retreatment with at least 1 additional cycle of 177Lu-DOTATATE. To be eligible for retreatment, patients were also required to have had a minimum response of stable disease after the initial treatment.
Initial treatment was defined as the initial regimen of up to 4 cycles of 177Lu-DOTATATE received by each patient; retreatment was defined as any additional cycles of 177Lu-DOTATATE given after the patient progressed after the initial treatment. As there is currently no set standard for selecting patients for retreatment with 177Lu-DOTATATE, the rationale for mandating at least a 6-mo gap between the initial treatment and retreatment cycles was to ensure that primary-resistant patients did not receive additional cycles of 177Lu-DOTATATE. If a patient received any further cycles after a second gap of at least 6 mo accompanied by evidence of progression, these were defined as additional retreatments.
The index date was the date of the patient’s first-ever treatment with 177Lu-DOTATATE. The index retreatment date was the date of the first retreatment cycle of 177Lu-DOTATATE. Patients were followed from the index date to death, loss to follow-up, or the end of the study period (June 30, 2021), whichever came first. All patients were required to have at least 6 mo of data available pre- and postindex.
This study was performed in compliance with the U.S. Health Insurance Portability and Accountability Act and the Declaration of Helsinki. The institutional review board (BRANY) approved this retrospective study, and the requirement to obtain informed consent was waived.
Institutional 177Lu-DOTATATE Protocol
All procedures were performed in the outpatient setting. Thirty minutes before administration of 177Lu-DOTATATE, patients received an infusion of 1,000 mL of 15% Clinisol (Baxter Healthcare Corp.) for kidney protection; this was continued for 4 h (250 mL/h). Patients received approximately 7.4 GBq (±10%) of 177Lu-DOTATATE via intravenous infusion over 30 min. Radiation exposure at 1 m at the time of discharge was 3–6 mR/h. Patients were administered antinausea medications before, during, and after therapy, as needed.
Outcomes and Measures
Patient characteristics were assessed at index and index retreatment. Laboratory values were also measured at the end of each treatment phase (from 2 wk before to 8 wk after the date of the last initial treatment cycle and the last retreatment cycle). Treatment patterns were measured over the initial and retreatment periods and included the number of 177Lu-DOTATATE cycles received, administered 177Lu-DOTATATE dose, time from initial treatment to retreatment, and other treatments received preindex.
Clinical outcomes were measured postindex and postindex retreatment. Treatment response, defined as the best overall response to 177Lu-DOTATATE treatment after the index and index retreatment dates, was evaluated according to RECIST 1.1. PFS was defined as the time from index or index retreatment until the date of progression or death from any cause. OS was defined as the time from index or index retreatment until death from any cause.
Hematologic laboratory parameters were compared before and after each treatment phase (e.g., before the first cycle vs. after the last cycle of initial treatment and retreatment) and at the start of each treatment phase (e.g., start of initial treatment vs. start of retreatment) to identify any significant differences. Toxicity was defined using the Common Terminology Criteria for Adverse Events, version 5.0. Adverse events (AEs) and serious AEs were recorded after the index and index retreatment dates. Key hematologic AEs were considered to be leukopenia, anemia, neutropenia, and thrombocytopenia.
Statistical Analysis
Patient characteristics and outcomes were evaluated descriptively. Continuous variables were reported using means and SDs or medians and interquartile ranges. Categoric variables were reported using counts and percentages.
Kaplan–Meier curves were used to evaluate PFS and OS. Patients were censored at loss to follow-up or the end of the study period. Hematologic parameters before and after each treatment phase and at the start of each treatment phase were compared using paired t tests. P values of less than 0.05 were considered to indicate statistical significance. All statistical analyses were performed in SAS, version 9.4 (SAS Institute Inc.).
RESULTS
Patient Characteristics
A total of 33 patients received retreatment with 177Lu-DOTATATE at the Excel Diagnostics and Nuclear Oncology Center; 31 met all of the eligibility criteria and were included in the study. The median follow-up was 3.3 y (interquartile range, 2.5–4.5 y) from the start of initial treatment and 1.0 y (interquartile range, 0.5–1.7 y) from the start of retreatment.
Included patients had a mean age of 60 ± 9 y at the start of initial treatment, 19 (61%) were male, and 29 (94%) were White (Table 1). Most patients (52%) had pancreatic NETs; the remaining patients had gastrointestinal NETs (39%), lung NETs (6%), and adrenal NETs (3%). At the start of the initial treatment, the mean number of metastatic sites was 3.1. The most common metastatic site was the liver (97%), followed by the bone (58%) and distant lymph nodes (58%). At the start of retreatment, 13 patients (42%) had emergent sites of metastases, most which were bone metastases (84%). All patients had liver metastases, and 61% had distant lymph node metastases.
Patient Characteristics at Initial Treatment and Retreatment with 177Lu-DOTATATE
Treatment Patterns
Among the 31 patients in the study, 30 had evidence of at least 1 treatment (whether surgical or medical) before their first 177Lu-DOTATATE cycle and 23 (74%) had 3 or more prior treatments. Most patients (81%) had received somatostatin analogs before 177Lu-DOTATATE, 52% had received targeted therapy (e.g., everolimus or sunitinib), whereas 48% had received cytotoxic chemotherapy (e.g., capecitabine and temozolomide or other agents). In addition, 68% of patients had a prior surgical resection, and 52% had undergone hepatic artery embolization. No patient in the study received any other treatment between their initial 177Lu-DOTATATE treatment and retreatment.
The average time from primary diagnosis to the start of initial treatment was 6.2 ± 5.3 y and to retreatment was 8.5 ± 5.8 y. Overall, patients received a median of 6 cycles (range, 5–8 cycles) of 177Lu-DOTATATE. The most common treatment pattern (68%) was 4 initial treatment cycles and 2 retreatment cycles (Table 2). The average time between the end of the initial treatment and the start of retreatment was 22 ± 14 mo. The average administered activity (including both treatment phases) was 41.9 ± 4.4 GBq. Two patients also went on to receive additional retreatment (1 and 2 cycles, individually) after a second period of at least 6 mo and progression after retreatment.
177Lu-DOTATATE Treatment Distribution by Treatment Phase
Effectiveness Outcomes
Best responses of partial response and stable disease were observed in 11 patients (35%) and 20 patients (65%) after initial treatment and 7 patients (23%) and 14 patients (45%) after retreatment, respectively (Table 3). The mean time to partial response was 7.7 ± 12.2 mo after the initial treatment and 5.7 ± 4.4 mo after retreatment. The corresponding times to stable disease were 2.8 ± 2.4 mo and 3.3 ± 2.6 mo. No patient had a complete response to either the initial treatment or retreatment. Notably, responses after retreatment were similar to those after the initial treatment, with most patients having a partial response or stable disease.
Best Overall Response to 177Lu-DOTATATE After Initial Treatment and Retreatment
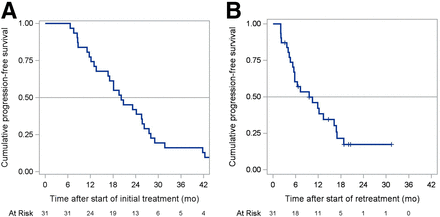
The median PFS after the start of the initial treatment was 20.2 mo (95% CI, 13.5–25.8 mo) and after the start of retreatment was 9.6 mo (95% CI, 5.5–16.2 mo) (Fig. 1). After the initial treatment, 23% of patients progressed or died within 1 y and 58% did so by 2 y. After retreatment, 58% of patients progressed or died within 1 y and 91% did so by 2 y.
PFS from start of initial treatment (A) and retreatment (B) with 177Lu-DOTATATE. PFS was calculated postindex and postindex retreatment.
The median OS after the start of the initial treatment was 42.6 mo (95% CI, 31.2–53.8 mo) and after the start of retreatment was 12.6 mo (95% CI, 9.6–18.9 mo) (Fig. 2). After the initial treatment, 0% of patients died within 1 y, and by 2 y, 16% had died. After retreatment, 45% of patients died within 1 y, and by 2 y, 87% had died. At the end of the follow-up period, 26 patients (84%) had died, whereas 5 (16%) were still alive.
OS from start of initial treatment (A) and retreatment with 177Lu-DOTATATE. OS was calculated postindex and postindex retreatment.
Safety Outcomes
Hematologic parameters decreased significantly during both the initial treatment and retreatment but recovered such that there were few significant differences between the values before the initial treatment and before retreatment (Table 4; Supplemental Table 1; supplemental materials are available at http://jnm.snmjournals.org). Among the key hematologic AEs, only 1 grade 3 AE was noted during the initial treatment (neutropenia). During retreatment, 4 grade 3 AEs were noted (1 leukopenia, 1 anemia, 2 thrombocytopenia) (Table 5). Clinically significant hematotoxicity occurred in 1 and 3 patients after the initial treatment and retreatment, respectively. No grade 3 or 4 nephrotoxicity (based on creatinine levels) was observed at any time during the study. No AEs of any grade were observed during additional retreatment in the 2 patients who received this.
Comparison of Hematologic Parameters at Start of Each Treatment Phase
AEs During Treatment with 177Lu-DOTATATE
DISCUSSION
This retrospective study included 31 patients with NETs who were retreated with 177Lu-DOTATATE at the Excel Diagnostics and Nuclear Oncology Center in Houston, Texas, during 2010–2021. To our knowledge, this is the first study to evaluate the real-world outcomes of retreatment with 177Lu-DOTATATE among patients with NETs in the U.S. Overall, the results indicate that retreatment with 177Lu-DOTATATE is feasible, effective, and safe.
Most (61%) of the patients in our cohort were male, and 94% were White. This was a heavily pretreated population, with approximately 3 of 4 having received 3 or more forms of treatment before their initial 177Lu-DOTATATE cycle. Overall, patients received a median of 6 cycles of 177Lu-DOTATATE (4 initial treatment cycles and 2 retreatment cycles), and the mean administered activity was 41.9 ± 4.4 GBq. The mean time from the end of initial treatment to the start of retreatment was 21.9 ± 12.8 mo.
Stable disease was the most common response after both the initial treatment (65%) and retreatment (45%). The median PFS after the initial treatment was 20.2 mo and after retreatment was 9.6 mo. The corresponding OS durations were 42.6 and 12.6 mo. Although the effectiveness results after retreatment may at first appear worse than those after the initial treatment, it is important to consider the smaller number of cycles administered during the retreatment phase, the greater disease burden of patients at index retreatment, and the necessary survival bias given that these are the same patients.
Hematologic and renal toxicity are considered the major side effects of radiolabeled somatostatin analogs. However, although we observed a significant decrease in the patients’ hematologic parameters during both the initial treatment and retreatment, these values recovered such that there were few differences between the values before the initial treatment and before retreatment. Other laboratory values were not significantly affected during the observed follow-up period.
Overall, our findings are consistent with those of previous studies, primarily conducted in Europe, in showing that additional retreatment cycles of 177Lu-DOTATATE are well tolerated and can offer disease-control benefits in patients with progressive NETs. Our study population was similar to that of previous studies in terms of demographics and disease and treatment characteristics (21–23,26–28). In addition, most patients’ having stable disease as their best response after retreatment is consistent with previous findings (21–23,26,27,29).
The objective response rate in our study at both the initial treatment and retreatment was somewhat higher than that in the phase 3 NETTER-1 trial (36% and 23% vs. 18%) (24). However, the median PFS from the initial 177Lu-DOTATATE treatment (20.2 mo) was lower than that in the NETTER-1 trial (28.4 mo). This may reflect the real-world patient population included in our study—patients who generally have a higher comorbidity burden than those included in clinical trials. Importantly, the distribution of NET subtypes also differed between our study and the NETTER-1 trial. Moreover, when considering the number of therapies received by patients in our study before initiating 177Lu-DOTATATE, as well as the fact that patients did not receive any other therapies between the initial treatment and retreatment with 177Lu-DOTATATE, it is likely that 177Lu-DOTATATE treatment was received late in their treatment journey. Compared with the PFS in other real-world studies, the median PFS we observed from index retreatment (9.6 mo) was longer than that reported by Rudisile et al. (22) (6 mo) but shorter than that reported by some other European studies (range, 14–22 mo) (21,23,27,28). The median OS in our study was marginally shorter than that reported in previous studies of retreatment (21–23,27,28).
Consistent with the results of previous studies, retreatment with 177Lu-DOTATATE demonstrated a good safety profile with minimal grade 3 or 4 AEs (22,23,26). Overall, our findings demonstrate that additional retreatment cycles of 177Lu-DOTATATE are well tolerated and can offer disease-control benefits in patients with progressive NETs. These results will be of value to both payers and providers treating patients who have often exhausted their available treatment options.
Limitations
The most important limitation of this study is the small number of patients. Nonetheless, 177Lu-DOTATATE retreatment is rare, and few data are available, so our study, to the best of our knowledge, is actually one of the largest on this topic to date and the first to be performed in a U.S. population. Despite our efforts to minimize bias in this study, several potential sources of selection bias may exist. First, this was a single-center study. Second, access to retreatment with 177Lu-DOTATATE in the U.S. is not universal because of limited reimbursement, and it is therefore possible that included patients who received retreatment with 177Lu-DOTATATE had specific disease- or patient-related characteristics that rendered them eligible for retreatment. Given the retrospective study design, there were also limitations related to the availability of data in the patients’ medical records. For example, some laboratory variables were available for only some patients. Finally, the follow-up duration may have been too short to capture all possible AEs, and long-term AEs associated with 177Lu-DOTATATE, including myelodysplastic syndrome and acute leukemia, were not available; therefore, we were not able to report on these outcomes.
CONCLUSION
To the best of our knowledge, this is the first U.S. study to evaluate the real-world effectiveness and safety of retreatment with 177Lu-DOTATATE in patients with advanced NETs. Retreatment with 177Lu-DOTATATE after progression appeared to be well tolerated and offered disease control in patients with progressive NETs after initial 177Lu-DOTATATE treatment.
DISCLOSURE
Ebrahim Delpassand receives consulting fees from Novartis, is employed by RadioMedix and the Excel Diagnostics and Nuclear Oncology Center, and has stock ownership in both RadioMedix and the Excel Diagnostics and Nuclear Oncology Center. Soheil Yazdi, Rodolfo Nunez, Afshin Shafie, and Susan Cork are employees of the Excel Diagnostics and Nuclear Oncology Center. Afshin Shafie reports stock ownership in Moderna. Clare Byrne and Jackson Tang report consulting fees from Novartis. Shashank Ghantoji, Antonio Nakasato, Corinne Strickland, and Jeetvan Patel are employees of Novartis and have stock ownership. No other potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: What is the real-world effectiveness and safety of retreatment with 177Lu-DOTATATE in patients with progressive NETs in the U.S.?
PERTINENT FINDINGS: In a single-center retrospective chart review study of 31 patients with advanced NETs who received retreatment with 177Lu-DOTATATE, the median PFS and OS after retreatment were 9.6 and 12.6 mo, respectively. 177Lu-DOTATATE demonstrated a good safety profile with few grade 3 or 4 AEs, and we did not observe any grade 3 or 4 nephrotoxicity during the initial treatment or retreatment with 177Lu-DOTATATE.
IMPLICATIONS FOR PATIENT CARE: Retreatment with 177Lu-DOTATATE after progression appeared to be well tolerated and offered disease control in patients with progressive NETs after initial 177Lu-DOTATATE treatment.
ACKNOWLEDGMENTS
We thank Asclepius Analytics for writing assistance.
Footnotes
Published online Mar. 21, 2024.
- © 2024 by the Society of Nuclear Medicine and Molecular Imaging.
REFERENCES
- Received for publication April 27, 2023.
- Revision received February 13, 2024.