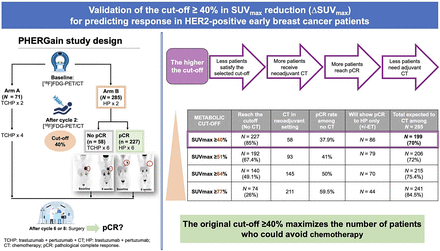
Visual Abstract
Abstract
The PHERGain trial investigated the potential of metabolic imaging to identify candidates for chemotherapy deescalation in human epidermal growth factor receptor 2 (HER2)–positive, invasive, operable breast cancer with at least 1 breast lesion evaluable by [18F]FDG PET/CT. [18F]FDG PET/CT responders were defined as patients with an SUVmax reduction (ΔSUVmax) of at least 40% in all of their target lesions after 2 cycles of trastuzumab and pertuzumab (HP) (with or without endocrine therapy). In total, 227 of 285 patients (80%) included in the HP arm showed a predefined metabolic response and received a total of 8 cycles of HP (with or without endocrine therapy). Pathologic complete response (pCR), defined as ypT0/isN0, was achieved in 37.9% of the patients. Here, we describe the secondary preplanned analysis of the best cutoff of ΔSUVmax for pCR prediction. Methods: Receiver-operating-characteristic analysis was applied to look for the most appropriate ΔSUVmax cutoff in HER2-positive early breast cancer patients treated exclusively with neoadjuvant HP (with or without endocrine therapy). Results: The ΔSUVmax capability of predicting pCR in terms of the area under the receiver-operating-characteristic curve was 72.1% (95% CI, 65.1–79.2%). The optimal ΔSUVmax cutoff was found to be 77.0%, with a 51.2% sensitivity and a 78.7% specificity. With this cutoff, 74 of 285 patients (26%) would be classified as metabolic responders, increasing the pCR rate from 37.9% (cutoff ≥ 40%) to 59.5% (44/74 patients) (P < 0.01). With this optimized cutoff, 44 of 285 patients (15.4%) would avoid chemotherapy in either the neoadjuvant or the adjuvant setting compared with 86 of 285 patients (30.2%) using the original cutoff (P < 0.001). Conclusion: In the PHERGain trial, an increased SUVmax cutoff (≥77%) after 2 cycles of exclusive HP (with or without endocrine therapy) achieves a pCR in the range of the control arm with chemotherapy plus HP (59.5% vs. 57.7%, respectively), further identifying a subgroup of patients with HER2-addicted tumors. However, the original cutoff (≥40%) maximizes the number of patients who could avoid chemotherapy.
Human epidermal growth factor receptor 2 (HER2) is overexpressed in approximately up to 20% of breast cancers and correlates with poor differentiation and high proliferation rates (1). Even though introduction of HER2-targeted therapies has led to a significant improvement in outcomes, a significant proportion of HER2-positive (HER2+) patients with early breast cancer (EBC) still suffer from recurrence and death.
Neoadjuvant chemotherapy has been the standard treatment for patients with locally advanced breast cancer because of its significant downstaging of primary tumors and the enhanced probability of successful breast-conserving surgery. Moreover, pathologic complete response (pCR) to neoadjuvant treatment has been consistently associated with better long-term survival outcomes, allowing the accelerated approval of new agents on the basis of this surrogate marker (2–4). In patients with HER2+ EBC, a sequence of anthracyclines and taxanes combined with trastuzumab has been the standard treatment for many years. More recently, it was observed in high-risk patients that a HER2 dual blockade with trastuzumab and pertuzumab (HP) as an adjuvant therapy increases pCR rates and improves invasive disease-free survival (iDFS) (5). Consequently, the landscape of neoadjuvant therapy has rapidly evolved from trastuzumab-based to dual HER2 blockade with HP-based regimens.
At the time of the initiation of the PHERGain study (6), all HER2+ patients with EBC received aggressive chemotherapy with anti-HER2 therapy in either the neoadjuvant or the adjuvant settings and were, therefore, exposed to significant toxicities including a small, but real, risk of cardiomyopathy. Predicting treatment response could lead to integration of deescalation strategies for the management of HER2+ patients with EBC and prevent unnecessary toxicities to those patients who achieve a pCR.
[18F]FDG PET/CT has been reported to be a useful tool to differentiate responding from nonresponding patients with EBC according to the changes of [18F]FDG uptake under neoadjuvant treatment (7,8). Recently, [18F]FDG PET/CT has also been shown to predict pathologic response to neoadjuvant therapy with the parameter of relative changes in [18F]FDG PET/CT after 1 or 2 cycles of treatment (9,10). The best time point for prediction seems to be after the second cycle of neoadjuvant therapy.
According to these results, we designed the PHERGain trial in which we investigated the potential of metabolic imaging ([18F]FDG PET/CT) to identify candidates with stage I–IIIA, operable, HER2-addicted tumors who might avoid chemotherapy (6). The study met its first coprimary endpoint, confirming that [18F]FDG PET/CT identifies patients with HER2+ EBC who were likely to benefit from chemotherapy-free dual HER2 blockade with HP.
In the PHERGain trial, [18F]FDG PET/CT responders were defined as those patients with an SUVmax reduction (ΔSUVmax) of at least 40% in all of their target lesions after 2 cycles of HP (with or without endocrine therapy). Here, we describe the results of a secondary preplanned analysis of the best cutoff of ΔSUVmax for pCR prediction.
MATERIALS AND METHODS
Study Design and Participants
The design and main results of PHERGain, an open-label, randomized, phase II trial (EudraCT: 2016-002676-27, ClinicalTrials: NCT03161353), have been previously reported (Fig. 1) (6). Details are shown in the supplemental materials (supplemental materials are available at http://jnm.snmjournals.org). This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of Hospital Arnau de Vilanova Valencia on March 31, 2017 (acta number 06/12017; last update on July 11, 2019). Written informed consent was obtained from all individual participants included in the study.
PHERGain study design. PHERGain assesses potential of metabolic imaging to identify candidates for chemotherapy deescalation in HER2+, stage I–IIIA, invasive, operable breast cancer with at least 1 breast lesion evaluable by [18F]FDG PET/CT. Asterisk indicates patients with hormone receptor–positive tumors who received endocrine therapy concomitantly with HP (except those receiving chemotherapy). Number symbol indicates patients who were RECIST responders after cycle 2 with SUVmax reduction ≥ 40%. Dagger represents patients who obtained pCR in breast and axilla (ypT0/isN0). Encircled areas indicate decreased lesion uptake after treatment cycles. Etx = endocrine therapy (letrozole for postmenopausal women and tamoxifen for premenopausal women; adjuvant ETx up to 3 y from surgery); R = randomization; TCHP = trastuzumab, pertuzumab, docetaxel, and carboplatin.
[18F]FDG PET/CT
Whole-body [18F]FDG PET/CT scans were obtained at baseline and after 2 cycles of study treatment. Two independent reviewers at 2 imaging laboratories who were masked to the patient characteristics, treatment allocation, and clinical outcome data performed image analysis and central quality assurance.
Target lesions from either breast or lymph nodes fulfilled both of the following criteria: an SUVmax of at least 1.5 cm times an SUVmean of the liver plus 2 SDs, and a longest diameter of at least 1.5 cm by MRI or ultrasound. Patients were considered metabolic responders in PHERGain when all target lesions showed a ΔSUVmax from baseline of at least 40% for all target lesions with no metabolic progression of nontarget lesions (6). Details about [18F]FDG PET/CT and the procedures are shown in the supplemental materials.
RESULTS
Patient Characteristics
Clinicopathologic baseline characteristics of patients avoiding chemotherapy (arm B) by pCR status are shown in Supplemental Table 1. Most of the characteristics for metabolic responders in arm B were not significantly different among patients with or without a pCR. Importantly, the median level of SUVmax at baseline was not significantly different between those patients achieving pCR (SUVmax, 8.8; interquartile range, 5.6–15.5) and those not achieving pCR (SUVmax, 10.8; interquartile range, 6.8–15.4; P = 0.248). However, the median level of ΔSUVmax after 2 cycles of treatment was 77.8% (interquartile range, 67.0%–85.4%) and 63.3% (interquartile range, 54.8%–74.8%) in patients with pCR and without pCR, respectively (P < 0.001) (Supplemental Table 1).
Baseline characteristics from patients in arm B, categorized by [18F]FDG PET/CT responder and nonresponder, are shown in Supplemental Table 2.
The rate of patients with a HER2 immunohistochemistry status of 3+ was greater in [18F]FDG PET/CT responders than in [18F]FDG nonresponders (83.3% vs. 67.2%, P = 0.006) (Supplemental Fig. 1).
ΔSUVmax Capability of Predicting pCR Among Metabolic Responders in Arm B
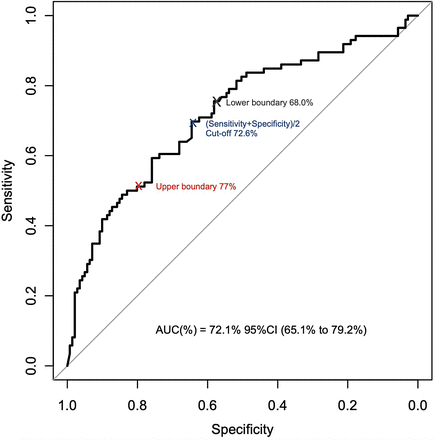
The receiver-operating-characteristic analysis yielded an area under the curve of 72.1% (95% CI, 65.1%–79.2%). The study, therefore, met the target predictive capability for a ΔSUVmax at 6 wk because the lower boundary of the 95% CI for the area under the curve was greater than 63.9% (Fig. 2).
Receiver-operating-characteristic curve of sensitivity and specificity values to predict pCR for different cutoffs for ΔSUVmax at 6 wk among metabolic responders in arm B. AUC = area under curve.
The best cutoff for the ΔSUVmax at 6 wk to predict pCR in metabolic responders was 72.6% (95% CI, 68.0%–77.2%), which was in the range of a median ΔSUVmax for metabolic responders (72.6%–69.6%) (Supplemental Table 3). The optimal ΔSUVmax cutoff was 77.2% to approximately 77%, with a 51.2% sensitivity and a 78.7% specificity. It would increase the rate of patients achieving a pCR in the group, avoiding the need for chemotherapy in comparison with patients in the lower boundary of the CI (59.5% vs. 41.1%, P = 0.007) (Table 1).
Association Between ΔSUVmax Cutoff and Chemotherapy Treatment in Arm B
The correlation between the 2 reviewers was 97.4% (95% CI, 96.7%–98.0%) (Supplemental Fig. 2). The calculation method of the ΔSUVmax 95% CI is described in Supplemental Table 3.
The baseline characteristics of patients with a metabolic response defined with the 77% ΔSUVmax cutoff in arm B are shown in Supplemental Table 4. Compared with the previous 40% ΔSUVmax cutoff, the subpopulation of metabolic responders identified with the 77% ΔSUVmax cutoff presented slightly higher percentages of patients older than 50 y (63.5% vs. 52.5%, P = 0.004), as well as HER2 3+ status (89.2% vs. 81.1%, P = 0.007) or HER-negative (41.9% vs. 30.8%, P = 0.05) breast cancer.
Association Between ΔSUVmax Cutoff and Adjuvant Chemotherapy for Patients from Arm B
The rate of patients who received neoadjuvant chemotherapy was 74.0% (211/285 patients; 95% CI, 68.5%–79.0%) with a ΔSUVmax cutoff of at least 77% and was 20.4% (58/285 patients; 95% CI, 15.8%–25.5%) with a ΔSUVmax cutoff of at least 40% (P < 0.001) (Table 1).
The rate of patients who needed adjuvant chemotherapy and did not receive neoadjuvant chemotherapy was 40.5% (30/74 patients; 95% CI, 29.3%–52.5%) with a ΔSUVmax cutoff of at least 77% and was 62.1% (141/227 patients; 95% CI, 55.5%–68.4%) with a ΔSUVmax cutoff of at least 40% (P = 0.001). Likewise, the rate of patients with pCR was 59.5% (44/74 patients; 95% CI, 47.4%–70.7%) for a ΔSUVmax cutoff of at least 77% and was 37.9% (86/227 patients; 95% CI, 31.6%–44.5%) with a ΔSUVmax cutoff of at least 40% (P = 0.001). Consequently, the rate of patients avoiding chemotherapy either in the neoadjuvant or in the adjuvant settings using the PHERGain strategy was 15.4% (44/285 patients; 95% CI, 11.4%–20.2%) with a ΔSUVmax cutoff of at least 77% and was 30.2% (86/285 patients; 95% CI, 24.9%–35.9%) with a ΔSUVmax cutoff of at least 40% (P < 0.001) (Table 1).
Association Between ΔSUVmax Cutoff and Clinical Outcomes in Arm A and Among Metabolic Responders and Nonresponders in Arm B
The pCR rate in patients treated with neoadjuvant chemotherapy in arm A (41/71 = 57.7%) was significantly higher than the pCR rate achieved in patients for whom chemotherapy was initiated after 2 cycles of HP (arm B metabolic nonresponders, 15/58 = 25.9%) or in patients not receiving neoadjuvant chemotherapy (arm B metabolic responders, 86/227 = 37.9%).
However, the patients with a ΔSUVmax cutoff of at least 77% who avoided neoadjuvant chemotherapy had a pCR rate similar to that of the patients treated with neoadjuvant chemotherapy in arm A (59.5% vs. 57.7%, P = 0.834) (Fig. 3).
pCR rate for patients with ΔSUVmax higher than or equal to cutoff values in arm B compared with pCR rate in whole arm A. P values compare pCR rate for arm B at different cutoffs vs. pCR rate for whole arm A (reference). Imputed and raw ΔSUVmax for arm B are presented. We reported higher P values for both analyses. In [18F]FDG PET nonresponders for arm B (blue line), there were no patients with ΔSUVmax ≥ 40% and only 8 patients had ΔSUVmax between 35% and 40%. We reported pCR for patients with ΔSUVmax ≥ 26% (29.4%; 5/17 patients). Line was interpolated between 26% and 40% cutoffs. Of note, each data point contains pCR rate for patients with ΔSUVmax ≥ cutoff. *P < 0.05. **P ≤ 0.01. ns = P > 0.05.
Interestingly, in arm A (n = 71), in which the [18F]FDG PET was performed under the combination of chemotherapy and dual HER2 blockade, the same 40% cutoff was used to discriminate patients with a low or high pCR rate: 1 of 10 patients (10%) and 40 of 61 patients (65.6%) reached pCR with a ΔSUVmax of less or more than 40%, respectively (P = 0.013).
DISCUSSION
Several neoadjuvant trials (TBCRC023, TRAINII, TBCRC026, and PAMELA) have evaluated the possibility to deescalate systemic therapy in HER2+ breast cancer patients on the basis of the assumption that a proportion of them is overtreated with current treatment regimens (15–17).
The PHERGain trial explored the deescalation strategy in a distinctive way on the basis of 3 different arguments. First, it explored a chemotherapy-free regimen, namely HP. Second, it was a strategy-based study with an original randomized and adapted design: the control arm A evaluated the pCR rate in patients receiving the standard therapy (chemotherapy with HP) and compared that rate with the rate in arm B, the deescalating arm in which patients received dual HER2 blockade alone (HP) (with or without endocrine therapy) for 6 wk. Continuation of the HER2-targeted therapy alone was conditional on a metabolic response on [18F]FDG PET/CT defined as a ΔSUVmax of at least 40%. If this definition was not reached, a standard chemotherapy was added to the HER2 dual blockade before surgery. Third, in contrast with other deescalating trials, the PHERGain trial included as a primary endpoint not only pCR but also the 3-y iDFS and enrolled a subgroup of patients who did not receive chemotherapy in either the neoadjuvant or the adjuvant settings (6,18).
[18F]FDG PET/CT has already been studied to monitor early metabolic changes in HER2+ tumors treated with neoadjuvant therapy and their correlation with pCR (19,20). However, finding the thresholds for relative changes in [18F]FDG uptake to distinguish between responding and nonresponding tumors is challenging and depends on the purpose of the imaging biomarker: higher thresholds are better suited to identify excellent responders, which is clinically meaningful in situations in which it is important to select patients with tumors highly sensitive to the treatment of interest, whereas lower thresholds are more adequate to identify nonresponders, which becomes highly relevant in cases of toxic or expensive treatments. When the 40% cutoff in the PHERGain trial was used, a total of 227 of 285 patients (80%) included in the HP arm met the criteria of metabolic response and 86 of the patients had a pCR (37.9%): the primary endpoint was met with rejection of the null hypothesis of a pCR no greater than 20% (6).
The purpose of the current preplanned analysis was to retrospectively identify better potential cutoff values for pCR prediction. We found this cutoff to be at least 77% with an improvement in pCR rate from 37.9% to 59.5%. Interestingly, this result is similar to that of the pCR rate achieved in the control arm A, in which all patients received chemotherapy (6). At the patient level, this 77% cutoff ensures the highest rate of pCR under HP, which should translate into an excellent iDFS without the addition of chemotherapy after surgery. However, at a global level, it is the 40% cutoff that allows chemotherapy sparing before or after surgery for the largest proportion of patients (30.2% instead of 15.4%). Overall, the redefined 77% cutoff would allow for the identification of patients with more HER2-addicted tumors, characterized by higher pCR rates and, possibly, superior prognoses.
It should be noted that a lower cutoff value for ΔSUVmax will expose more patients to salvage therapy: this is less of a concern nowadays, following the striking results of the KATHERINE trial, which showed an 11.3% absolute improvement in the 3-y iDFS rate for 14 cycles of the antibody–drug conjugate trastuzumab emtansine compared with results of 14 cycles of trastuzumab when given to women not achieving a pCR after standard neoadjuvant chemotherapy and anti-HER2 treatment (mostly trastuzumab). KATHERINE has contributed to the perception that not achieving a pCR is not too worrisome: however, the overall survival results of that study still have to be reported (21). Of note, trastuzumab emtansine was not the standard treatment at the time PHERGain was launched.
The only study apart from PHERGain that evaluated a chemotherapy-free regimen with HP was the TBCR0256 trial, and that included 83 patients with low [18F]FDG-avid tumors (21,22). The duration of targeted therapy was 12 wk with a metabolic response evaluation at 2 wk (compared with a 24-wk duration of targeted therapy and 6 wk for response evaluation in PHERGain), and chemotherapy could be introduced at any time at investigator discretion, whereas this was allowed in PHERGain only when a lack of response was documented. After a pCR of 32% was reported (close to the 37.9% pCR reported in chemotherapy-free patients from PHERGain), the TBCRC026 trial did not meet its primary endpoint of an area under the curve greater than 0.65 for the correlation between a change in lean body mass–corrected SUV and pCR.
Interestingly, the TBCRC026 investigators concluded that 40% was the optimal cutoff for the change in lean body mass–corrected SUV. On the other hand, they claimed that an absolute lean body mass–corrected SUV of less than 3 on day 15 could also be an excellent predictor of pCR, a biomarker that is strongly correlated, at the patient level, with improved long-term outcomes (15,21). However, we believe this measurement could be subjected to variations in real-world settings and recommend using ΔSUVmax. A weakness of the PHERGain trial could be the 6-wk therapy window before [18F]FDG PET/CT response evaluation: according to TBCRC026, an earlier time point could have been more appropriate.
Another neoadjuvant anti-HER2 regimen investigated as a chemotherapy-free option is trastuzumab combined with lapatinib (with or without endocrine therapy). TBCRC023 and PAMELA investigators developed a multiparameter classifier to predict the probability of pCR under this regimen. They found that a pCR of at least 50% correlated with a combination of a HER2 fluorescence in situ hybridization ratio of at least 4.6, HER2 immunohistochemistry 3+ positive cells greater than 97.5%, a HER2-enriched prediction analysis microarray 50 subtype, and the absence of PIK3CA mutations (22). Although the need for prospective validation remains, these results suggest that combining baseline and dynamic biomarkers, such as [18F]FDG PET/CT response, could be the most efficient strategy for selecting patients who can avoid chemotherapy.
To our knowledge, the AVATAXHER phase II study is the only neoadjuvant trial apart from PHERGain with a response-adapted design performed in 142 patients with HER2+ EBC (intention-to-treat population), but the goal was a treatment escalation under insufficient response to chemotherapy plus trastuzumab. The AVATAXHER study aimed to predict pCR by [18F]FDG PET/CT early and investigate whether adding bevacizumab could improve the pCR rate in patients who were unlikely to respond to initial treatment (19). The predicted responders to [18F]FDG PET/CT (ΔSUVmax ≥ 70%; n = 69) after 1 treatment cycle continued receiving standard docetaxel plus trastuzumab. Predicted nonresponders (ΔSUVmax < 70%; n = 73) were randomized (2:1) to receive docetaxel, trastuzumab, and bevacizumab or the same treatment. The pCR rate from PET responders in breast and axilla was 53.6%. Adding bevacizumab in nonresponders increased the pCR rate (43.8% vs. 24.0%; P value unspecified). Interestingly, a retrospective analysis of the AVATAXHER trial found an optimal cutoff of 76%, similar to the one reported in our study (22).
CONCLUSION
An increased SUVmax cutoff (≥77%) after 2 cycles of exclusive HP achieved a pCR in the range of the control arm with chemotherapy plus HP in the PHERGain trial (59.5% vs. 57.7%, respectively), selecting a subgroup of patients with HER2-addicted tumors. However, the original cutoff (≥40%) maximizes the number of patients who could avoid chemotherapy, maintaining excellent 3-y iDFS rates. Higher SUVmax cutoffs may better identify patients who would be candidates for treatment-escalation strategies. For this reason, the clinical applicability of the cutoff will depend on the aim of the clinical trial and still needs to be further investigated.
DISCLOSURE
Geraldine Gebhart is on the advisory board for Roche, receives research funding from Roche paid to her institution, and has an immediate family member who received personal fees from Roche. Marleen Keyaerts reports institutional funding from Precirix and personal stocks and shares from Abscint. Manuel Ruiz-Borrego reports research support from Novartis and participates on an advisory board of Novartis. Santiago Escriva-de-Romani was an invited speaker and serves on an advisory board for Daiichi Sankyo/AstraZeneca, Roche, and Seagen and is a local principal investigator with institutional financial interests in Byondis, Lilly, MedSIR, Roche, and Synthon. Marco Colleoni reports honoraria from Novartis; an advisory board role with Pierre Fabre, Pfizer, OBI Pharma, and Celldex; and a research grant from Roche. Manuel Atienza de Frutos reports employment from Lilly as well as stocks and shares in Lilly. Aleix Prat reports honoraria from Pfizer, Novartis, Roche, MSD Oncology, Lilly, Daiichi Sankyo, and Amgen; consulting roles with Amgen, Roche, Novartis, Pfizer, Bristol-Myers Squibb, Boehringer Ingelheim, PUMA Biotechnology, Oncolytics Biotech, Daiichi Sankyo, Abbvie, and NanoString Technologies; research funding to the institution from Roche, Novartis, Incyte, and PUMA Biotechnology; intellectual property (PCT/EP2016/080056; WO/2018/096191); travel expenses from Daiichi Sankyo; other funding from Oncolytics and Peptomyc; and an immediate family member who is an employee of Novartis. Peter Schmid reports honoraria from Pfizer, AstraZeneca, Novartis, Roche, Merck, and Boehringer Ingelheim; a consulting role with Pfizer, AstraZeneca, Novartis, Roche, Merck, Boehringer Ingelheim, Bayer, Eisai, Celgene, and Puma; and a grant to the institution from Roche, Genentech, Oncogenex, and Novartis. Sofia Braga reports consulting and advisory board roles with Daiichi Sankyo, AstraZeneca, Novartis, and Roche; speaker and conference fees from Daiichi Sankyo, AstraZeneca, Novartis, and Roche; and travel fees from Daiichi Sankyo, AstraZeneca, Novartis, and Roche. Miguel Sampayo-Cordero reports involvement with the advisory board and the speaker’s bureau of, and funding from, MEDSIR and Optimapharm and employment with MEDSIR. Javier Cortés reports consulting and advisory fees from Roche, Celgene, Cellestia, AstraZeneca, Seattle Genetics, Daiichi Sankyo, Erytech, Athenex, Polyphor, Lilly, Merck Sharp & Dohme, GSK, Leuko, Bioasis, Clovis Oncology, Boehringer Ingelheim, Ellipses, Hibercell, BioInvent, Gemoab, Gilead, Menarini, Zymeworks, Reveal Genomics, and Expres2ion Biotechnologies; honoraria from Roche, Novartis, Celgene, Eisai, Pfizer, Samsung Bioepis, Lilly, Merck Sharp & Dohme, and Daiichi Sankyo; research funding to the institution from Roche, Ariad Pharmaceuticals, AstraZeneca, Baxalta GmbH/Servier Affaires, Bayer Healthcare, Eisai, F. Hoffman-La Roche, Guardanth Health, Merck Sharp & Dohme, Pfizer, Piqur Therapeutics, Puma C, and Queen Mary University of London; stock in MEDSIR, Nektar Pharmaceuticals, and Leuko (owned by a relative); and travel and accommodation expenses from Roche, Novartis, Eisai, Pfizer, Daiichi Sankyo, AstraZeneca, and Gilead. José Manuel Pérez-García reports an advisory role with Lilly, Roche, Eisai, Daichii Sankyo, AstraZeneca, Seattle Genetics, and Gilead; travel expenses from Roche; and employment with MEDSIR. Antonio Llomart-Cussac reports research support from Roche, Agendia, Lilly, Pfizer, Novartis, Merck Sharp & Dohme, Gilead, and Daichii Sankyo; a consulting or advisory role with Lilly, Roche, Pfizer, and Novartis; sitting on the speakers’ bureaus for Lilly, AstraZeneca, and Merck Sharp & Dohme; receiving travel support from Roche, Pfizer, and AstraZeneca; and owning stock or having other ownership in MEDSIR and Initia-Research. Petra Gener reports employment with MEDSIR. No other potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: What was the optimal [18F]FDG PET/CT cutoff value identified in the PHERGain trial for predicting pCR in HER2+ EBC patients treated with neoadjuvant trastuzumab and pertuzumab?
PERTINENT FINDINGS: The study found an optimized cutoff of at least 77% for ΔSUVmax, leading to an improved pCR rate of 59.5% compared with 37.9% achieved with the original cutoff of at least 40%. Although the higher cutoff offers a higher rate of pCR under HP alone, the original cutoff allows a larger proportion of patients (30.2%) to avoid chemotherapy.
IMPLICATIONS FOR PATIENT CARE: With the optimized cutoff, a significant number of patients could potentially avoid unnecessary chemotherapy, offering personalized and tailored treatment approaches for improved patient care.
Footnotes
Published online Apr. 4, 2024.
- © 2024 by the Society of Nuclear Medicine and Molecular Imaging.
REFERENCES
- Received for publication July 21, 2023.
- Revision received February 16, 2024.