Visual Abstract
Abstract
The application of prostate-specific membrane antigen (PSMA)–targeted α-therapy is a promising alternative to β−-particle–based treatments. 211At is among the potential α-emitters that are favorable for this concept. Herein, 211At-based PSMA radiopharmaceuticals were designed, developed, and evaluated. Methods: To identify a 211At-labeled lead, a surrogate strategy was applied. Because astatine does not exist as a stable nuclide, it is commonly replaced with iodine to mimic the pharmacokinetic behavior of the corresponding 211At-labeled compounds. To facilitate the process of structural design, iodine-based candidates were radiolabeled with the PET radionuclide 68Ga to study their preliminary in vitro and in vivo properties before the desired 211At-labeled lead compound was formed. The most promising candidate from this evaluation was chosen to be 211At-labeled and tested in biodistribution studies. Results: All 68Ga-labeled surrogates displayed affinities in the nanomolar range and specific internalization in PSMA-positive LNCaP cells. PET imaging of these compounds identified [68Ga]PSGa-3 as the lead compound. Subsequently, [211At]PSAt-3-Ga was synthesized in a radiochemical yield of 35% and showed tumor uptake of 19 ± 8 percentage injected dose per gram of tissue (%ID/g) at 1 h after injection and 7.6 ± 2.9 %ID/g after 24 h. Uptake in off-target tissues such as the thyroid (2.0 ± 1.1 %ID/g), spleen (3.0 ± 0.6 %ID/g), or stomach (2.0 ± 0.4 %ID/g) was low, indicating low in vivo deastatination of [211At]PSAt-3-Ga. Conclusion: The reported findings support the use of iodine-based and 68Ga-labeled variants as a convenient strategy for developing astatinated compounds and confirm [211At]PSAt-3 as a promising radiopharmaceutical for targeted α-therapy.
Metastatic castration-resistant prostate cancer is a disease with poor prognosis and median survival times no longer than 12 mo (1–3). However, the recent approval by regulatory agencies of the prostate-specific membrane antigen (PSMA)–targeted radiopharmaceutical [177Lu]Lu-PSMA-617 (Pluvicto; Novartis) is raising hope (4). Nevertheless, [177Lu]Lu-PSMA-617 is able to treat only 50%–60% of late-stage metastatic castration-resistant prostate cancer patients (5–7). It has been shown that PSMA-targeted α-radiotherapy is an effective and promising alternative to β−-based approaches (8,9). For example, the application of 225Ac-labeled PSMA ligands resulted in positive outcomes for patients who did not respond to 177Lu-based therapy (10,11).
One of the most promising α-emitters is 211At. Its production is scalable, and its relatively short half-life (7.2 h) matches the tumor retention time of many targeting vectors (12). Radionuclides with half-lives that fit the biological tumor retention time of the targeting vector, such as 211At, should optimize the dose that can be delivered, maximizing the therapeutic effect (13). In addition, using nuclides with half-lives shorter than that of, for example, 225Ac can result in a lower radiation burden on nontarget tissues. In addition, efforts have been made to explain the relative ineffectiveness of therapeutic fibroblast activation protein inhibitor derivatives using longer-lived radionuclides such as 177Lu and 90Y (14,15). Thus, it is not surprising that several 211At-based radiopharmaceuticals targeting PSMA have been developed (16–19).
In this work, a class of 211At-labeled PSMA-targeting structures based on the successful glutamate–urea–lysine construct was developed. The main aim was to possibly reduce deastatination and mimic the biodistribution pattern of PSMA-617, including its ability to internalize into cells—a relevant feature, because cell internalization has been shown to positively correlate with therapeutic effect for PSMA-targeting radiopharmaceuticals (20,21). Figure 1 displays the design rationale of the compounds. The glutamate–urea–lysine construct was chosen to promote specificity and nanomolar affinity toward PSMA. Aromatic amino acids in the linker region were inserted to promote internalization via lipophilic interaction with the S1 aromatic region deep inside the PSMA binding pocket (22). It was also chosen to 211At-astatinate the inhibitors within this lipophilic linker region, because it was hypothesized that this could shield 211At from oxidative species, which are supposed to be responsible for deastatination processes (23). Consequently, achievement of higher in vivo stability was expected using this labeling approach. Finally, the DOTA chelator was preserved in the structures to promote excretion because of the intrinsic polarity of this moiety (24) and to have the possibility of radiolabeling with 68Ga, which allowed more convenient in vitro and in vivo evaluation. Therefore, 68Ga-labeled iodine-based surrogates were evaluated to approximate the in vivo behavior of their 211At-labeled counterparts. This strategy was used because astatine does not exist in a nonradioactive form; in addition, its scarce availability would have made the evaluation studies even more challenging.
Design rationale of reported series. Compound selected as lead candidate, [211At]-PSAt-3, is used as example. EuK = glutamate–urea–lysine; PK = pharmacokinetics.
MATERIALS AND METHODS
Chemistry and Radiochemistry
The series of synthesized compounds is depicted in Figure 2. The chemical synthesis of all derivatives was performed via standard solid phase peptide synthesis (SPPS) or solution phase peptide synthesis (25). All building blocks for peptide chemistry were from commercial sources, except for silyl-containing amino acid 9, which was synthesized using palladium-mediated chemistry (26). The precursor for [68Ga]Ga-PSMA-617 was obtained from commercial sources. Figure 3 outlines the general synthetic pathway for amino acid 9 and precursors 18 and 11. Details on the syntheses and purifications are available in the supplemental materials (supplemental materials are available at http://jnm.snmjournals.org). Labeling with 68Ga was performed using standard procedures; details are available in the supplemental materials.
Series of compounds synthesized for preliminary evaluation through their 68Ga-labeled versions. Note presence of cold iodine in various strategic positions. EuK = glutamate–urea–lysine.
(A) Synthetic pathway toward key amino acid 9 (Fmoc-3TMS-Phe). (a) AcCl, methanol, 80°C, 5 h (i); Boc2O, Et3N, tetrahydrofuran, room temperature (r.t.), overnight (o.n.) (ii). (b) Pd2(dba)3, Me3Si2, water, KHCO3, meCgPPh, dimethylformamide, 100°C, 72 h. (c) LiOH, tetrahydrofuran/water, r.t., 1.5 h (i); 4 M HCl, dioxane, r.t., 30 min (ii). (d) Fmoc-Cl, 10% NaHCO3(aqueous)/dioxane, 0°C, 2 h. (B) Synthesis and subsequent radiolabeling of lead candidate precursors PSGa-3 and PSTMS-3. (e) Standard solid-phase peptide synthesis: hexafluorophosphate azabenzotriazole tetramethyl uronium, N,N-diisopropylethylamine, dimethylformamide, 35°C, 60 min to o.n. (i); 20% piperidine in dimethylformamide, 35°C, 10 min (ii); trifluoroacetic acid–water–triisopropylsilane (94:3:3), r.t., 2 h, or 4 M HCl, dioxane, r.t., 2 h (iii). (f) 68Ga3+, (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid) (1.0 M, pH 4.0), 95°C, 5 min. (g) 211At, methanol, trifluoroacetic acid, 70°C, 10 min (i); 10 mM natGa(NO)3 in 0.1 M HCl, NaOAc buffer (pH 4.5), 70°C, 10 min (ii).
211At was produced on a Scanditronix MC32 cyclotron (Copenhagen University Hospital) via the 209Bi(α,2n)211At reaction. Thereafter, 211At was isolated through dry distillation (Atley C100; Atley Solutions) and recovered in chloroform for further processing.
For 211At labeling, 211At in chloroform (50–200 MBq) was poured into a V-shaped glass vial, and solvent was evaporated with a flow of nitrogen. Afterward, a solution of the trimethylsilane precursor (precursor 18, PSTMS-3) in methanol was added (5 µL, 50 nmol), followed by the addition of N-chlorosuccinimide (400 µg, 3.0 µmol) in 20 µL of methanol and a 0.3 M methanol–acetic acid solution (20 µL). The whole mixture was then evaporated to dryness with a gentle nitrogen flow. Thereafter, trifluoroacetic acid (20 µL) was added to the mixture and heated to 70°C for 10 min (27). Directly after labeling, the mixture was evaporated to dryness and redissolved in sodium acetate buffer (80 µL, pH 5.5), followed by addition of an excess of natGaNO3. The solution was heated to 70°C for an additional 10 min to ensure complete complexation. After radiolabeling and cold complexation, the mixture was diluted to 200 µL by the addition of an acetonitrile–water–trifluoroacetic acid mixture (20:80:0.1) and subsequently purified by high-performance liquid chromatography. Collected fractions containing [211At]PSAt-3-Ga were diluted with water (10 mL) and loaded onto a preconditioned hydrophilic lipophilic balanced cartridge (Chromafix HLB Small 60 µm; Macherey-Nagel). A solid phase extraction cartridge was washed with water (5 mL), dried with air, and [211At]PSAt-3-Ga–eluted with 0.5 mL of absolute ethanol. The ethanol was evaporated under a stream of nitrogen, and the compound was reconstituted in phosphate-buffered saline (pH 7.4) containing 5% ethanol.
Cell Culture and In Vitro Assays
For in vitro and in vivo evaluation of the compounds, the PSMA-positive cell line LNCaP was used (CRL-17400; American Type Culture Collection). To this end, cells were cultured in RPMI medium supplemented with 10% fetal calf serum, 1% sodium pyruvate, and 1% penicillin–streptomycin to avoid bacterial growth. Cells were kept at 37°C in humidified air supplemented with 5% carbon dioxide; when a confluence of at least 80% was reached, cells were harvested using trypsin–ethylenediaminetetraacetic acid and subsequently processed. Cells were regularly authenticated (last authentication April 2023) and were Mycoplasma-free.
Internalization, plasma protein binding, lipophilicity, and binding affinity assays were performed as described previously (28). Details on the methodologies are provided in the supplemental materials.
In Vivo Evaluation
For PET imaging, 7- to 8-wk-old BALC/c nu/nu mice (Janvier) were subcutaneously inoculated on their right flank with 5 × 106 LNCaP cells (in a 1:1 ratio of phosphate-buffered saline to Matrigel; Corning). Once the tumor size was about 1 cm3, mice were injected via the tail vein with 500 pmol of the 68Ga-labeled compounds (10–15 MBq). A dynamic PET scan spanning 0–60 min after injection was recorded (30 frames), followed by a nontriggered localizer and a 3-step, whole-body, T1-weighed, 3-dimensional MR scan (PET/MR 3T; Bruker BioSpin). In addition, static images (10-min scan time) were recorded at 2 h after injection, also followed by a nontriggered localizer and a 3-step, T1-weighed, 3-dimensional MR image. PET/MR data were obtained via ParaVision version 3.0 (ParaVision) and reconstructed through a maximum-likelihood expectation maximization algorithm at 0.5 mm for 18 iterations. MR-based attenuation correction was applied to the reconstruction.
For biodistribution of [211At]PSAt-3-Ga, the same tumor model was used. Once the tumors were sizable, mice were injected via the tail vein with approximately 100 kBq of [211At]PSAt-3-Ga. Mice were killed at 2, 6, and 24 h after injection (n = 4–6 per time point), and organs of interest were harvested. Organs included heart, lungs, liver, salivary glands, thyroid (trachea), spleen, stomach, small intestine, and kidneys; blood, tail, and grafted LNCaP tumor were also harvested. After weighing, each organ was measured in an automated γ-counter (Hidex AMG) with an energy window of 60–100 keV. The percentage injected dose per gram of tissue (%ID/g) was calculated using the amount of solution injected and the animal or organ weight. All counts were decay-corrected and standardized to the injection solution.
All animal experiments were performed according to the directive 2010/63/EU of the European Parliament and the European Council on the protection of animals used for scientific purposes and approved by the Danish Animal Experiments Inspectorate under an approved animal license (2021-15-0201-01041, approved December 2021, biodistribution studies), as well as the Federal Republic of Germany, Regional Council Freiburg (35-9185.81/G18/04, approved February 2018, PET/MR studies). In all cases, mice were housed upon arrival in groups to acclimatize them for a week with a light-to-dark period of 12:12 h and under controlled environmental conditions. Access to fresh water and standard pellet diet was provided ad libitum.
Statistics
All experiments were performed at least in triplicate, with the exception of PET/MRI (n = 1). All multiple measurement results are expressed as mean ± SD. Data were analyzed and plotted using Prism (version 8.0.1; GraphPad Software). Applicable P values were determined by Student t test and were considered significant when smaller than 0.05.
RESULTS
Chemistry
Reference Compounds
Eight PSMA iodine-bearing derivatives (PSGa-2 through PSGa-9) were successfully synthesized (Fig. 2). These compounds were used as surrogates to study the pharmacokinetic behavior of their corresponding 211At-labeled counterparts. They were synthesized according to reported procedures (25,28), with overall yields of 2%–15%. In all cases, the key intermediate was the dissolved or resin-supported lysine–urea–glutamate construct, and syntheses were performed using single-peptide coupling steps that led to overall yields of 4%–15%, typical for SPPS procedures. More details on the syntheses and analytic data are shown in the supplemental materials.
Precursor
Synthesis of silyl precursor 18 was successful in a 6-step SPPS sequence with an overall yield of 2%. The key intermediate, nonnatural amino acid 9, could be obtained in an overall yield of 10% over 4 steps. Figure 3 displays the synthetic strategy. Details on the synthetic procedures, along with analytic data, are shown in the supplemental materials.
Radiochemistry
Labeling with 211At was performed through trimethylsilyl-bearing precursors (27). The radiolabeling of precursor 18 was successful, with a radiochemical conversion of 75% and a radiochemical yield of 35% after natGa3+ complexation and high-performance liquid chromatography purification. 68Ga labeling was performed following conditions described in the supplemental materials and resulted in radiochemical conversions of at least 98% for all iodine-bearing analogs. Radiochemical conversion and radiochemical yield were determined according to consensus guidelines (29).
In Vitro Evaluation
Physicochemical properties such as lipophilicity (logDpH7.4 in n-octanol and phosphate-buffered saline) of the 68Ga-labeled derivatives and plasma protein binding were determined, with results in the expected ranges. These are summarized in Table 1.
In Vitro Characterization Data of 68Ga-Labeled Surrogate Compounds (n = 3)
Internalization studies of the 68Ga-labeled derivatives showed specific uptake within LNCaP cells (Table 1) after 45 min of incubation. Uptake was specific as proven by blocking experiments with 2-phosphonomethyl pentanedioic acid. Binding affinities were in the nanomolar range for all tested 68Ga-labeled inhibitors (Table 1). [68Ga]Ga-PSGa-4 or [68Ga]Ga-PSGa-5 and [68Ga]Ga-PSGa-6 showed an inhibitor constant 2-fold lower than that of [68Ga]Ga-PSMA-617. Other compounds displayed binding affinities similar to that of [68Ga]Ga-PSMA-617 (P > 0.05). With regard to internalization, [68Ga]Ga-PSGa-6 internalization was significantly lower than that of [68Ga]Ga-PSMA-617 (P = 0.046), whereas the rest of the tested compounds showed nonsignificant differences compared with [68Ga]Ga-PSMA-617 (P > 0.05).
PET/MRI
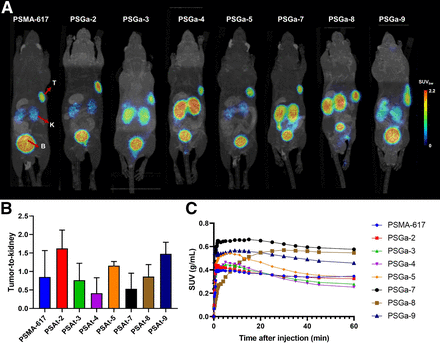
PET imaging in LNCaP xenograft mice revealed fast tumor uptake accompanied by rapid clearance through renal pathways for all 68Ga-labeled compounds. All tracers showed uptake higher than or similar to that of [68Ga]Ga-PSMA-617, with peak tumor accumulation of 0.4–0.6 SUV (g/mL) within 0–60 min after injection. Tumor uptake slightly decreased over time, resulting in tumor retention at 2 h after injection of 0.2–0.5 SUV (g/mL), with the highest SUV for [68Ga]Ga-PSGa-9. The highest tumor uptake was observed for [68Ga]Ga-PSGa-7, with SUVmax (g/mL) of 0.7 at 15 min after injection. High imaging contrast (tumor-to-muscle ratios of 3.6–6.6 at 1 h after injection) could be detected at early time points (Fig. 4). PET scan data showed the best tumor accumulation values for compounds [68Ga]Ga-PSGa-7 to [68Ga]Ga-PSGa-9; however, these compounds were accompanied by higher kidney retention. Compounds [68Ga]Ga-PSGa-2 to [68Ga]Ga-PSGa-5 showed marginally lower SUVs in the tumor but quicker renal clearance.
(A) PET/MRI maximum intensity projections 2 h after injection of 0.5 nmol of 68Ga-labeled surrogate compounds and reference PSMA-617. (B) Tumor-to-kidney ratios 2 h after injection. (C) Tumor accumulation (SUV) 0–60 min after injection in LNCaP nude mouse (BALB/c nu/nu) model (n = 1). B = bladder; K = kidney; T = tumor.
[211At]PSAt-3 Biodistribution
Figure 5 shows the biodistribution of [211At]PSAt-3-Ga in the LNCaP mouse xenograft (supplemental materials include a table with detailed values). Tumor enrichment of [211At]PSAt-3-Ga was high, with 19 ± 8 %ID/g at 1 h after injection. Uptake was reduced over 24 h to 7.6 ± 2.9 %ID/g. In comparison, the gold standard PSMA-617 showed similar pharmacokinetic behavior but with higher retention at 24 h after injection (supplemental materials). Deastatination occurred only to a minor extent, as exemplified by low uptake in organs such as the stomach (1.6 ± 0.4 %ID/g), spleen (3.1 ± 0.6 %ID/g), and thyroid or trachea (1.6 ± 1.1 %ID/g) at 1 h after injection. The clearance of [211At]PSAt-3-Ga was through renal pathways, with high kidney uptake at early time points (82 ± 15 %ID/g at 1 h after injection and 44 ± 8 %ID/g at 6 h after injection), followed by rapid washout over 24 h, leading to 8.0 ± 4.2 %ID/g. Uptake of [211At]PSAt-3-Ga in salivary glands was low (2.0 ± 0.7 %ID/g at 1 h after injection and 0.4 ± 0.1 %ID/g at 24 h after injection) and did not increase over time.
(A) Biodistribution (%ID/g) of [211At]PSAt-3-Ga in dissected organs (n = 4 for 2 h after injection; n = 6 for 6 and 24 h after injection). (B) Tumor-to-organ ratios (n = 4 for 2 h after injection; n = 6 for 6 and 24 h after injection). Detailed values in supplemental materials. p.i. = postinjection.
DISCUSSION
The compounds in the reported series are based on preclinically and clinically successful PSMA-targeting compounds that show sufficient tumor uptake, tumor retention, high cell internalization, and rapid excretion. The main goal of this study was to design radiopharmaceuticals that can accommodate a halogen and do so without causing a significant change in the pharmacokinetic profile of the radioligand while decreasing deastatination (Figs. 1 and 2). To this end, 2 strategies were followed. First, the aim was to replace or complement the 2-naphthyl-l-alanine aromatic system with iodine-bearing l-phenylalanine derivatives. It was hypothesized that this strategy would not significantly modify the binding and internalization properties of the ligands (30). Second, it is known that the 2-naphthyl-l-alanine aromatic system interacts with the accessory PSMA lipophilic site, improving binding (31). By placing the carbon–astatine bond in this lipophilic site, its stability could be potentially increased as its exposure to oxidative species was reduced (23). To modulate the polarity of the compounds and thereby influence their pharmacokinetic profile, especially with regard to their clearance, they were conjugated to a DOTA chelator. This addition allows imaging of surrogate compounds by replacing astatine with iodine—for example, labeling DOTA with 68Ga or 111In—whereas the astatinated versions could serve therapeutic purposes.
In this respect and for ease of handling, the compound series was initially evaluated as the 68Ga-labeled version. This served as a starting point for the full in vitro characterization of the inhibitors, as well as a preliminary pharmacokinetic study through PET imaging. In vitro evaluation of the 68Ga-labeled compounds involved internalization and competitive binding affinity assays. The results from the internalization assays showed that the chemical modifications did not affect the ability of the ligands to internalize, with one exception. [68Ga]Ga-PSGa-4 had a surprisingly low rate of internalization. The reason for this unexpected behavior is unknown. Furthermore, it was confirmed that the cyclohexyl moiety is essential to promote internalization. [68Ga]Ga-PSGa-6, which lacks this aliphatic cycle, showed very low uptake in PSMA-positive LNCaP cells. Binding affinities were in the same nanomolar range as for the gold standard [68Ga]Ga-PSMA-617. These findings were expected, because the binding affinity is mainly driven by the glutamate–urea–lysine construct (32). LogDpH7.4 determination confirmed the hydrophilic nature of the compounds. As expected, the slightly more lipophilic compounds PSGa-7 to PSGa-9 displayed higher binding toward plasma proteins than did their more hydrophilic counterparts (Table 1). As such, they were expected not only to result in marginally higher tumor uptake but also to be excreted somewhat more slowly (33).
Encouraged by these results, the 68Ga-labeled compounds (besides [68Ga]Ga-PSGa-6, because of its poor internalization rate) were progressed to PET imaging studies in an LNCaP mouse xenograft. Dynamic scans of the compounds were recorded 0–60 min after injection. For all structures, time–activity curves (Fig. 4; supplemental materials) revealed a fast pharmacokinetic profile with peak tumor accumulation of 0.4–0.7 SUV (g/mL) and excretion through renal pathways. As expected, clearance for [68Ga]Ga-PSGa-7 to [68Ga]Ga-PSGa-9 was slightly slower, along with narrowly higher tumor accumulation. Additional static images at 2 h after injection confirmed the pharmacokinetic behavior of the series (Figs. 4A and 4B). Tumor uptake and retention of the compounds were high for all candidates, with slightly increased values for [68Ga]Ga-PSGa-7 to [68Ga]Ga-PSGa-9. However, these compounds also showed lowered kidney-to-tumor ratios—most likely because of their slower clearance rate. Because high kidney retention has been shown to be detrimental for targeted α-radiotherapy (16), PSGa-7 to PSGa-9 were excluded from further studies using 211At. Because compounds PSGa-2, PSGa-3, and PSGa-5 showed similar pharmacokinetic profiles, similar internalization rates, and affinities in the same nanomolar order, it was decided to furnish [211At]PSAt-3. This decision was mainly driven by the synthetic accessibility of [211At]PSAt-3 and its precursor compared with PSAt-2 and PSAt-5 (supplemental materials provide detailed information).
The chosen evaluation strategy, based on the short-lived radionuclide 68Ga, can assess only early biodistribution time points, and as such, this strategy cannot predict the residence time of the tested compounds, which in turn is positively correlated with therapeutic outcomes. Because 211At also possesses a relatively short half-life, early biodistribution might be sufficient to select the most promising candidate for 211At labeling. Consequently, the trimethylsilyl-containing precursor 18 of [211At]PSAt-3 was synthesized. The choice of a trimethylsilyl over a stannyl-leaving group was mainly based on the higher stability of the silyl moiety compared with stannanes, as organotin compounds are sensitive to acidic conditions and cannot be easily applied to standard SPPS procedures (34). In contrast, the trimethylsilyl group allowed the introduction of amino acid 9 into standard SPPS procedures. Amino acid 9 is the key intermediate to introduce the silyl-based precursor moiety into precursor 18. However, the use of trimethylsilyl is not exempt of shortcomings, because its reactivity is relatively low toward electrophilic aromatic substitutions. Higher temperatures (around 70°C) in neat trifluoroacetic acid have to be used (27). As these conditions lead to cyclization of the unprotected glutamate–urea–lysine structural motif (35), a relatively complex product mixture was observed after radiolabeling of [211At]PSAt-3 (supplemental materials). However, [211At]PSAt-3 was successfully isolated in sufficient radiochemical yield (35%) and radiochemical purity (≥98%).
To mimic the pharmacokinetic behavior of [68Ga]Ga-PSGa-3, natGa3+ was chelated into the DOTA moiety of [211At]PSAt-3. Omission of the metal in the chelator moiety can have an effect on the pharmacokinetic profile of the ligand (36). Afterward, biodistribution studies of [211At]PSAt-3-Ga were performed in an LNCaP xenograft model and revealed that [211At]PSAt-3-Ga exhibited favorable tumor-targeting properties (Fig. 5), as evidenced by tumor accumulation of 19 ± 8 %ID/g at 1 h and 7.6 ± 2.9 %ID/g at 24 h after injection. These values are comparable to those reported for other 211At-labeled PSMA-targeting compounds (17). For both [211At]PSAt-3-Ga and the previously reported compounds, tumor retention decreases around 50% at 24 h after injection compared with that of [177Lu]Lu-PSMA-617 (supplemental materials) when a similar dose (∼100 kBq) is administered to the tumor-bearing mice. A potential explanation for the decrease in tumor uptake would be the effect of an α-emitter, such as 211At, on tumor cells compared with the weaker β-emitter. A lower dose of 211At might already be sufficient to eradicate some tumorous cells and therefore decrease the %ID/g at 24 h after injection. Such effectivity of α-emitters has been proven with similar single doses of 225Ac-labeled radiopharmaceuticals (37,38). In addition, kidney retention for [211At]PSAt-3-Ga compared with [177Lu]Lu-PSMA-617 is similar at early time points, albeit slightly higher at 24 h after injection. [211At]PSAt-3-Ga also showed low accumulation in organs related to deastatination, with a maximum of 3.6 ± 0.8 %ID/g in the thyroid at 6 h after injection. This is in line with the initial hypothesis that positioning the astatine in the PSMA lipophilic accessory pocket prevents deastatination, highlighting the need for careful design of astatinated radiopharmaceuticals to prevent deastatination. However, careful validation studies need to be performed to better assert deastatination, especially in animals such as mice, in which the small size of the deastatination organ markers could lead to increased errors in the %ID/g determinations.
CONCLUSION
In this study, a series of compounds was synthesized and evaluated in vitro and through PET imaging using 68Ga-labeled and iodine-substituted versions to identify a lead compound and then develop a 211At-labeled PSMA radiopharmaceutical. This lead compound was finally radiolabeled with 211At for biodistribution studies in an LNCaP xenograft model. [211At]PSAt-3-Ga demonstrated favorable distribution, with good tumor accumulation, adequate renal-mediated clearance, and negligible deastatination. The results highlight [211At]PSAt-3-Ga as a compound not inferior to the previously reported 211At-labeled PSMA-targeting compounds and emphasize the need for a head-to-head comparison using the same animal model to identify the best candidate. Nevertheless, the findings herein described establish [211At]PSAt-3-Ga among the leading candidates for PSMA-targeted α-radiotherapy and preclinical efficacy, as well as comparison studies with other compounds or radionuclides that are under way.
DISCLOSURE
This work received financial support from Deutsche Forschungsgemeinschaft (423813989/GRK2606) and from the Independent Research Fund Denmark (1032-00177B). Matthias Herth, Andreas Kjaer, Andreas Jensen, Ann-Christin Eder, and Matthias Eder are inventors on a patent application for the compounds described herein. No other potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: Can clinically relevant PSMA-targeting compounds be used as a good platform for developing an α-emitting inhibitor, with their similar favorable pharmacokinetic properties?
PERTINENT FINDINGS: The rationally designed [211At]PSAt-3-Ga showed a satisfactory pharmacokinetic profile with good tumor uptake and negligible nontarget tissue accumulation.
IMPLICATIONS FOR PATIENT CARE: If proven effective in controlling tumor growth or eradicating tumors in preclinical models, [211At]PSAt-3-Ga could be translated into clinical settings for application in targeted α-therapy. In addition, this work strengthens the theses of bringing 211At into clinical practice as a better and more efficient alternative to current α-emitting radionuclides.
ACKNOWLEDGMENTS
We thank Lisa Domogalla for technical assistance during the PET/MRI study. The graphical abstract was created with BioRender (Science Suite).
Footnotes
- © 2024 by the Society of Nuclear Medicine and Molecular Imaging.
REFERENCES
- Received for publication November 15, 2023.
- Revision received January 23, 2024.