Visual Abstract
Abstract
The immune-fibrosis axis plays a critical role in cardiac remodeling after acute myocardial infarction. Imaging approaches to monitor temporal inflammation and fibroblast activation in mice have seen wide application in recent years. However, the repeatability of quantitative measurements remains challenging, particularly across multiple imaging centers. We aimed to determine reproducibility of quantitative inflammation and fibroblast activation images acquired at 2 facilities after myocardial infarction in mice. Methods: Mice underwent coronary artery ligation and sequential imaging with 68Ga-DOTA-ECL1i to assess chemokine receptor type 2 expression at 3 d after myocardial infarction and 68Ga-FAPI-46 to assess fibroblast activation protein expression at 7 d after myocardial infarction. Images were acquired at 1 center using either a local or a consensus protocol developed with the second center; the protocols differed in the duration of isoflurane anesthesia and the injected tracer dose. A second group of animals were scanned at the second site using the consensus protocol. Image analyses performed by each site and just by 1 site were also compared. Results: The uptake of 68Ga-DOTA-ECL1i in the infarct territory tended to be higher when the consensus protocol was used (P = 0.03). No difference was observed between protocol acquisitions for 68Ga-FAPI-46. Compared with the local protocol, the consensus protocol decreased variability between individual animals. When a matched consensus protocol was used, the 68Ga-DOTA-ECL1i infarct territory percentage injected dose per gram of tissue was higher on images acquired at site B than on those acquired at site A (P = 0.006). When normalized to body weight as SUV, this difference was mitigated. Both the percentage injected dose per gram of tissue and the SUV were comparable between sites for 68Ga-FAPI-46. Image analyses at the sites differed significantly, but this difference was mitigated when all images were analyzed at site A. Conclusion: The application of a standardized acquisition protocol may lower variability within datasets and facilitate comparison of molecular radiotracer distribution between preclinical imaging centers. Like clinical studies, multicenter preclinical studies should use centralized core-based image analysis to maximize reproducibility across sites.
The immune-fibrosis axis has emerged as a prognostic imaging biomarker and therapeutic target for the progression of heart failure after acute myocardial infarction (1). For example, radiotracer molecular imaging of chemokine receptors, including C-C chemokine receptor type 2 (CCR2), provides a sensitive indication of local inflammation after myocardial injury that correlates with the severity of functional decline (2). Similarly, fibroblast activation protein (FAP) imaging reveals transient upregulation of activated fibroblasts early after myocardial infarction and predicts the subsequent functional outcome (3–5).
However, given the technical specialization involved in image acquisition, reconstruction, and analysis, obtaining reproducible quantitative values for radiotracer uptake can be challenging. Few studies have compared the reproducibilities of quantitative small-animal radiotracer images acquired at different imaging facilities. Mannheim et al. reported widely different whole-body biodistributions of 18F-FDG in healthy mice when images were acquired and analyzed at 4 different imaging centers (6). Cardiac uptake alone varied by 120%. Even when variables were minimized using identical procedures, operators, and equipment, there remained considerable differences in quantitative cardiac 18F-FDG uptake (6). Importantly, such studies have focused on 18F-FDG, the uptake of which is governed by the metabolic state—which can be difficult to control, particularly under continuous anesthesia (7–9). The quantitative comparability of newer molecular imaging radiotracers across multiple laboratories has not been investigated. Given the growing emphasis on data integrity and reproducibility in preclinical research (10,11) and the rapid rollout of preclinical imaging studies, a direct assessment of the reproducibility of quantitative cardiac radiotracer uptake is desirable.
We hypothesized that a standardized acquisition protocol applied at 2 distinct imaging laboratories with cardiac expertise would reduce the variability in quantitative image analysis. We selected 2 preclinically established radioligands targeting aspects of the immune-fibrosis axis: 68Ga-DOTA-ECL1i, targeting CCR2 expressed by proinflammatory monocytes/macrophages, and 68Ga-FAPI-46, targeting FAP expressed by activated cardiac fibroblasts.
MATERIALS AND METHODS
Animals
Male C57BL/6N mice (n = 25) were purchased from Charles River Germany for site A. For site B, male and female CCR2+/gfp heterozygous mice (n = 4 of each) with a C57BL/6J background were used. In these mice, 1 CCR2 allele is replaced by green fluorescence protein, and these mice do not exhibit differences in inflammatory cell behavior relative to wild-type mice. Animals were housed in groups under temperature-controlled conditions and a 12-h light/12-h dark cycle, and a standard laboratory diet and water were freely available. All animal experiments were approved by the local state authority (Landesamt für Verbraucherschutz und Lebensmittelsicherheit or Washington University Institutional Animal Care and Use Committee) and conducted in accordance with European, U.S. (National Institutes of Health Office of Laboratory Animal Welfare), and international guidelines.
Surgery
Surgical myocardial infarction was induced at 8–10 wk of age at each site as described previously (1), with minor variations between sites. After analgesic pretreatment, mice were anesthetized, intubated, and mechanically ventilated. A left thoracotomy and pericardiotomy exposed the left anterior descending coronary artery, which was ligated ∼1 mm distal to the left auricle. The chest wall was then closed, anesthesia was discontinued, and animals recovered with continued postoperative analgesia treatment. Surgical details for each site are provided in Supplemental Table 1 (supplemental materials are available at http://jnm.snmjournals.org).
Study Design
At site A, animals were assigned to either the local acquisition protocol (n = 9) or the consensus acquisition protocol (n = 10). At site B, mice (n = 8) underwent imaging using the consensus protocol. Sequential imaging was performed to assess inflammation at 3 d after myocardial infarction using 68Ga-DOTA-ECL1i and fibroblast activation at 7 d after myocardial infarction using 68Ga-FAPI-46.
Radiosynthesis
68Ga-DOTA-ECL1i and 68Ga-FAPI-46 were produced as described previously (12,13), with minor deviations between centers, as noted in Supplemental Table 2. FAPI-46 precursor was provided by Sofie Biosciences. Radiochemical purity (>95%) was confirmed by high-performance liquid chromatography, and the product was diluted in isotonic saline for injection. The average cold doses administered were 0.92 ± 0.19 (mean ± SD) nmol for 68Ga-DOTA-ECL1i and 0.99 ± 0.64 nmol for 68Ga-FAPI-46.
Image Acquisition
Site A Local Protocol
PET and CT images were acquired in pairs using dedicated small-animal cameras (Inveon DPET and CT; Siemens) as described previously (1). Mice were anesthetized under isoflurane (2%; 0.8 L of O2/min), and a 27-gauge catheter was inserted into a lateral tail vein. 68Ga-DOTA-ECL1i (12.5 ± 0.8 MBq) or 68Ga-FAPI-46 (12.5 ± 0.6 MBq) was administered as a 150-μL bolus in heparinized saline. Animals then recovered on a heating pad in a holding cage to allow conscious distribution of the radiotracer. After 40 min, animals were reanesthetized under isoflurane and placed prone on the scanner bed (Minerve) with continuous warm air circulation and respiration monitoring. Images were acquired from 45–60 min after radiotracer administration. At the conclusion of the PET scan, a low-dose CT scan was acquired for radiotracer colocalization. To define the infarct size and location, 18F-FDG (18.3 ± 2.1 MBq) was administered as a 200-μL bolus by intraperitoneal injection subsequent to 68Ga image acquisition with the animal in the identical position of the scanner bed. A static image was acquired 20–30 min after 18F-FDG injection. Images were histogrammed to a single frame and reconstructed using an iterative ordered-subset expectation maximization (2 iterations) fast maximum a posteriori (18 iterations) algorithm with a target resolution of 1.5 mm and scanner-based corrections for scatter and dead time. A transmission scan with paired 57Co sources was used for attenuation correction.
Consensus Protocol
The consensus protocol followed the same sequence as the site A local protocol, with some exceptions. First, a lower administered dose was used for 68Ga-DOTA-ECL1i (8.5 ± 1.7 MBq) or 68Ga-FAPI-46 (7.9 ± 2.6 MBq). Second, animals were maintained under isoflurane anesthesia throughout the radiotracer uptake phase and for the full duration of the experiment. Third, a low-dose CT scan was used for attenuation correction rather than a transmission scan. All other parameters were kept identical. Images at site B were acquired using the consensus protocol and a Mediso dedicated small-animal PET/CT camera. Images were reconstructed using the manufacturer’s iterative TeraTomo 3DOSEM algorithm (6 subsets, 4 iterations). At site B, 18F-FDG was administered using the same intravenous catheter as for the 68Ga-labeled radiotracer administration, and images were acquired directly after the conclusion of the low-dose CT scan.
Image Analysis
At site A, regions of interest (ROIs) were drawn using 18F-FDG images and imported to colocalized 68Ga images as described previously (1). 18F-FDG and 68Ga images were coregistered by alignment to the common CT image. Minimal manual adjustment following the anatomy was required, because the anesthetized animal was not moved between acquisitions. Briefly, an ROI for viable noninfarcted myocardium was defined by interactive thresholding on the 18F-FDG scan and cross-applied to each matched 68Ga scan. The infarct territory was defined by manual placement of an ROI covering the full anterolateral wall without 18F-FDG uptake superior to the liver (Supplemental Fig. 1). With the colocalization CT image as a guide, additional ROIs were defined for the liver and quadriceps femoris skeletal muscle as nonspecific control regions. At site B, the infarct region was defined similarly, but a spheric ROI of fixed size (∼20 mm3) was manually positioned in the anterolateral wall or the inferoseptal wall.
Radiotracer uptake was quantified as the percentage injected dose per gram of tissue (%ID/g), where a uniform tissue density of 1 g/mL was assumed, and—to account for variable body weight—also as the SUV. Infarct size was calculated from polar map analysis using MunichHeart software (1). To assess whether variability in image analysis could be reduced, 2 individuals at site A analyzed imaging datasets from that site.
Statistics
All data are presented as mean ± SD. Statistical analysis was performed using GraphPad Prism (version 9.3). Quantitative radiotracer uptake was compared between imaging protocols or between sites using the Mann–Whitney test. Three groups for infarct sizes and body weights were compared using the Kruskal–Wallis test. Pearson product moment correlation was used to assess the relationship between parameters. Bland–Altman analysis was used to determine the intercenter and interoperator comparabilities of quantitative image analyses. Statistical significance was considered at a P value of less than 0.05.
RESULTS
Permanent Coronary Artery Ligation Generates Range of Scar Sizes at Different Imaging Sites
Infarct sizes were determined by polar map analysis of 18F-FDG images acquired at 3 d after coronary occlusion, where scar was defined as less than 60% of the normalized maximum signal. Animals at site A exhibited a median infarct extent of 22% of the left ventricle (range, 8%–41%). There was no statistical difference in infarct size between mice assigned to different acquisition protocols (Supplemental Fig. 2A). By contrast, site B infarct sizes tended to be modestly larger (median, 29%; range, 18%–40%), but the differences from the site A infarct sizes were not statistically significant. Although there was no difference in body mass between protocols at site A (24 ± 2 vs. 23 ± 1 g; P = 0.411), CCR2+/gfp mice at site B had lower body mass (18 ± 2 g; P < 0.001), which may have affected radiotracer biodistribution (Supplemental Fig. 2B).
Standardized Acquisition Protocol Limits Variability of Inflammation Imaging Signal at Site A
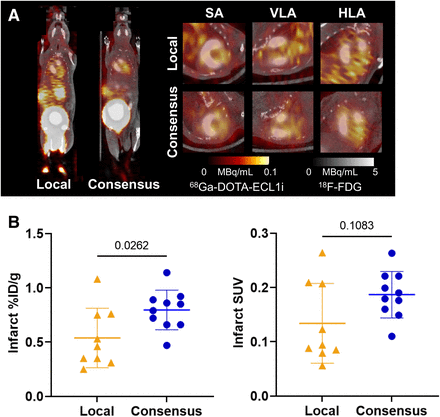
To determine the impact of standardized acquisition on quantitative image analysis, we first acquired images using either the local or the consensus protocol in mice after coronary artery occlusion. 68Ga-DOTA-ECL1i uptake was observed in the anterolateral nonviable infarct region of the left ventricle at 3 d after coronary artery occlusion (Fig. 1A). Quantitative analysis of the infarct region demonstrated significantly higher %ID/g and a trend toward higher SUVmean in mice scanned with the consensus protocol than in those scanned with the local protocol (Fig. 1B). Population variability in cardiac radiotracer uptake was lower among animals scanned with the consensus protocol than among those scanned with the local protocol (σ = 0.51 vs. 0.21) (Table 1). Radiotracer uptake values in nontarget organs, including remote noninfarcted myocardium, liver, and skeletal muscle, were similar in local and consensus protocol acquisitions (Supplemental Fig. 3).
(A) Representative cardiac 68Ga-DOTA-ECL1i images at 3 d after myocardial infarction and acquired using local and consensus imaging protocols. (B) Quantitative tracer uptake in infarct myocardium as %ID/g (left) and SUV (right) on images acquired using local or consensus acquisition protocol. Statistical analysis performed using Mann–Whitney U test. HLA = horizontal long axis; SA = short axis; VLA = vertical long axis.
Variability of Quantitative Tracer Uptake in Infarct Region
Standardized Acquisition Has Limited Impact on Quantification of Fibroblast Activation at Site A
The uptake of 68Ga-FAPI-46 at 7 d after coronary artery occlusion was generally elevated in the anterolateral infarct region and remote noninfarcted myocardium (Fig. 2A). Quantitative analysis demonstrated directly comparable radiotracer uptake in the infarct territory using either the local or the consensus protocol when calculated as %ID/g or SUVmean (Fig. 2B). The variabilities of the radiotracer signal in the infarct region were similar between protocols (σ = 0.33 vs. 0.31) (Table 1). In nontarget organs, the radiotracer signal was consistently low, although there was a tendency toward higher liver accumulation with the local protocol than with the consensus protocol (Supplemental Fig. 4).
(A) Representative cardiac 68Ga-FAPI-46 images at 7 d after myocardial infarction and acquired using local and consensus imaging protocols. (B) Quantitative tracer uptake in infarct myocardium as %ID/g (left) and SUV (right) in images acquired using local or consensus acquisition protocol. Statistical analysis performed using Mann–Whitney U test. HLA = horizontal long axis; SA = short axis; VLA = vertical long axis.
Standardized Acquisition Protocol Facilitates Direct Comparison of Quantitative Imaging Data Between Sites
To evaluate the consistency of imaging measurements across centers, 2 imaging sites acquired CCR2 and FAP PET images after coronary artery ligation using the consensus protocol, analyzed at site A. Radiotracer distributions were comparable, with localized cardiac accumulation of 68Ga-DOTA-ECL1i at 3 d and 68Ga-FAPI-46 at 7 d (Fig. 3A). A quantitative assessment revealed a consistently higher infarct region %ID/g CCR2 PET signal in images acquired at site B than in those acquired at site A using identical protocols (Fig. 3B). When uptake was normalized to body weight using SUVmean, the difference between protocols was dampened. A similar pattern was observed in other peripheral organs, including the quadriceps skeletal muscle. By contrast, semiquantitative uptake of infarct region 68Ga-FAPI-46 was more consistent between imaging sites using %ID/g and SUVmean (Fig. 3C). Distribution to other peripheral organs was unaffected by the acquisition site. This discrepancy could not be explained by differences in infarct size, as there was no correlation between 18F-FDG–defined infarct size and the intensity of the CCR2 PET signal (r = 0.205; P = 0.482) or the intensity of the FAP PET signal (r = 0.200; P = 0.494).
(A) Representative cardiac 68Ga-DOTA-ECL1i and 68Ga-FAPI-46 images acquired using consensus imaging protocol at site A and site B. (B) Quantitative uptake of 68Ga-DOTA-ECL1i in infarct myocardium as %ID/g (left) and SUV (right). (C) Quantitative uptake of 68Ga-FAPI-46 in infarct myocardium as %ID/g (left) and SUV (right). Statistical analysis performed using Mann–Whitney U test. HLA = horizontal long axis; SA = short axis; VLA = vertical long axis.
Image Quantification Is More Reproducible with Centralized Single-Site Analysis Than with Multisite Image Analysis
All images acquired at each site and with each protocol were analyzed independently by operators at site A and site B. Pairwise comparisons demonstrated consistently higher infarct territory %ID/g and SUVmean for CCR2 images analyzed at site B than for those analyzed at site A (Fig. 4A). Bland–Altman analysis identified a strong correlation between these images (r = 0.940; P = 0.001), with consistent bias between sites (−0.24 ± 0.11 %ID/g) (Supplemental Fig. 5A). For FAP images, there was more variability in pairwise comparisons, but the average radiotracer uptake tended to be calculated at a higher value at site B than at site A (Fig. 4B). Bland–Altman analysis demonstrated a smaller bias in the infarct territory FAP signal (−0.17 ± 0.18 %ID/g) (Supplemental Fig. 5B). By contrast, Bland–Altman analysis demonstrated a high correlation and a smaller bias in CCR2 and FAP images between operators at site A alone (Supplemental Fig. 6). Because the radiotracer signal was calculated as an average for the total volume of interest, we evaluated the consistency of the sizes of the analyzed volumes of interest between centers. The average size of the volume of interest was smaller at site B than at site A (28 ± 5 vs. 22 ± 1 mm3; P = 0.014); this finding may have contributed to the difference in the quantitative values (Supplemental Fig. 7). Notably, when image analysis was performed by 2 operators at site A, the quantitative values tended to be more similar, reflecting the similarity of the sizes of the volumes of interest for analysis.
(A) Comparison of quantitative 68Ga-DOTA-ECL1i uptake in infarct region on all images analyzed at site A or site B as %ID/g (left) or SUV (right). (B) Comparison of 68Ga-FAPI46 uptake in infarct region on all images analyzed at site A or site B as %ID/g (left) or SUV (right). Statistical analysis performed using Student’s paired t test.
DISCUSSION
The exponential growth of cardiac radionuclide-based molecular imaging applications, such as using inflammation- and FAP-targeted radiotracers, has generated extensive data; however, the lack of harmonized image acquisition and analysis procedures hinders comparison of data between studies, especially when undertaken by distinct imaging laboratories. Standardization of imaging protocols in clinical practice facilitates multicenter imaging trials, but implementation of similar procedures in small-animal imaging is lacking. With growing recognition of the importance of data reproducibility, there is an obligation in the imaging community to assess the impact of laboratory-imposed imaging protocols on quantitative measurements. Here, we compared distinct imaging protocols using 2 emerging molecular imaging markers of inflammation and fibroblast activation in the setting of experimental myocardial infarction in mice. This analysis revealed 3 key observations: first, changes in the image acquisition protocol regarding anesthesia duration and administered dose have a minimal impact on quantitative measurements acquired at the same institution; second, a harmonized imaging protocol reduces variability in images acquired in 2 different laboratories; and third, image analysis may represent a greater source of variability than image acquisition, suggesting a benefit to core lab–style centralized quantitative image analysis.
Although there has been widespread interest in the scientific community regarding the reproducibility of preclinical experiments, there remain hindrances to the adoption of standardized practices for data acquisition and analysis to facilitate interlaboratory comparisons. In molecular imaging, the confluence of biologic, radiochemical, and technical factors presents unique challenges to the comparability of quantitative measurements. Semiquantitative cardiac PET signals of chemokine receptor CXCR4 ligands after myocardial infarction diverge by up to 2-fold in %ID/g values (14–17). Likewise, the recent explosion of 68Ga-FAPI-46 preclinical experiments has generated wide ranges of quantitative uptake values in the myocardium (3,4,18,19). Although some of this variability may be ascribed to species, radiotracer, or camera differences, there is no standardization in the methods used for acquisition and analysis.
In the present study, we observed a significant difference in 68Ga-DOTA-ECL1i uptake in the infarct region between imaging protocols at the same site; this result was not apparent with 68Ga-FAPI-46. This finding may reflect differences in the temporal dynamics of inflammation and fibroblast activation; that is, earlier postinfarction imaging of inflammation may be more sensitive to a change in the acquisition protocol than later postinfarction imaging of fibrosis. This notion may reflect differences in tracer blood clearance between the 2 compounds or differences in the tracer substrate between mobile blood-derived leukocytes and myocardium-derived activated fibroblasts. Blood activity of 68Ga-DOTA-ECL1i also tends to be higher at 50–60 min after administration than that of 68Ga-FAPI-46, which may be influenced by continuous isoflurane anesthesia affecting spillover to the infarct region.
Desire for reproducible preclinical imaging data has stimulated comparison studies across imaging centers, but many previous studies focused solely on the performance of different scanners. Phantom studies of the 2 scanners used in the present study showed similar spatial resolutions in hot rods, with comparable uniformity and spillover ratios (20). Nonequal SUV suggested higher variation with filtered backprojection reconstruction, but the phantom studies did not assess iterative reconstruction (20). Filtered backprojection reconstruction poorly resolves the myocardium (8), necessitating iterative algorithms like those used in the present study to differentiate between the infarct and remote noninfarcted myocardium.
One multicenter preclinical imaging study addressed the variability of quantitative 18F-FDG biodistribution in healthy mice (6). Across 4 imaging laboratories, myocardial uptake of 18F-FDG was widely divergent across laboratories. Further, image acquisition by the same personnel using different protocols led to minor differences in cardiac 18F-FDG %ID/g (6). Metabolic preparation is crucial for 18F-FDG imaging, such that fluctuations in blood glucose affect myocardial glucose transport (7,8). In the present study, despite different camera systems and personnel, the variation in quantitative imaging was reduced by use of a common acquisition protocol.
A major factor contributing to the difference in quantitative imaging values between centers appears to derive from image analysis. Interoperator %ID/g was more consistent in the automatically defined remote noninfarcted myocardium defined by the threshold of the colocalized 18F-FDG image. Conversely, the infarct region required manual definition of boundaries, resulting in greater disparity of the ROI volume and quantitative uptake for both 68Ga-DOTA-ECL1i and 68Ga-FAPI-46. Although site A used the full region of the infarct territory for average radiotracer uptake, site B used a smaller region of consistent size across animals. The results suggest that restriction of the analysis area may emphasize regions of high radiotracer uptake that may not be representative of the entire infarct territory. By contrast, larger volumes of interest may lack sensitivity to subtle changes. Focal uptake of 68Ga-DOTA-ECL1i in the infarct territory was consistently higher when analyzed by site B than by site A, irrespective of the location or image acquisition protocol, whereas the FAP signal was more dispersed between analyses. This result may reflect the larger area of FAP upregulation after infarction, as demonstrated in clinical studies (5), making it less susceptible to regional sampling bias. The larger size of the infarct ROI also counteracts potential partial-volume effects, which are more pronounced with smaller structures. The wider affected area of FAP expression may also suggest a value to global myocardium quantification, although further study is warranted. Taken together, the comparability of quantitative molecular imaging measurements likely requires centralized core facility–based image analysis at a single center, similar to the model used in clinical studies (21). One advantage of standardized protocols across multiple sites is the ability to increase sample sizes in imaging studies, as exemplified in a recent MRI study of stroke across 6 centers in more than 2,500 animals (22). That study demonstrated the feasibility of standardized procedures and centralized analysis to facilitate preclinical multicenter evaluation of therapeutics.
Some limitations to the present study should be acknowledged. First, biologic factors could not be standardized between centers. Specifically, the relative infarct sizes tended to be larger in the smaller mice operated at site B as compared with site A. Larger infarct size is associated with a stronger inflammatory response (1), which could contribute to the difference in subsequent fibroblast activation (23). When analysis was restricted to only animals with infarct sizes of greater than 20% of the left ventricle, the same pattern of tracer uptake site to site was apparent for both CCR2 and FAP imaging. Moreover, the difference in mouse strains may have contributed to variable inflammation after myocardial infarction. Although the CCR2+/gfp mice exhibit similar inflammatory cell content compared with the wild type, the difference between C57BL/6N and C57BL/6J mice may influence postinfarction healing (24). Likewise, we cannot discount an influence of sex on the observed uptake patterns. Initial analysis suggests no difference in male and female uptake of either 68Ga-DOTA-ECL1i (P = 0.857) or 68Ga-FAPI-46 (P = 0.457), but dedicated analysis in both sexes has not yet been pursued. Second, differences in pain management in the first 1–5 d after surgical infarction, particularly the use at site B of carprofen, which has antiinflammatory activity, may affect leukocyte dynamics and infarct healing postinfarction. We are heartened that the imaging signal of 68Ga-DOTA-ECL1i remained comparable to mice at site A that were treated with the lesser antiinflammatory tramadol over the first days after infarction, suggesting that this action of carprofen postoperatively does not adversely impact healing. Third, because images at different sites were acquired using different commercial scanners, image reconstruction could not be completely standardized. Variability in the reconstruction algorithm can affect quantitative measurements (25), although the iterative reconstructions applied here have been widely validated against ex vivo measurements. Nonetheless, further investigation of multisite reproducibility of different reconstruction methods is warranted. Finally, we have only addressed in vivo imaging measurements without further validation by ex vivo autoradiography, γ-counting or protein measurements. It should be noted that correlation of in vivo image measurements have been directly correlated to the expression of CCR2 and FAP in prior studies (2,4).
CONCLUSION
The adoption of a common acquisition protocol may lower the variability of quantitative image analysis and facilitate comparison of radiotracer uptake between different imaging centers in preclinical imaging. Despite robust image acquisition, these findings suggest that multisite preclinical imaging studies would benefit from centralized image analysis, similar to clinical study design, to facilitate comparison of radiotracer uptake in images acquired at multiple sites.
DISCLOSURE
This work was supported by grants from the Leducq Foundation (to Robert J. Gropler, Frank M. Bengel, Yongjian Liu, and James T. Thackeray), the National Institutes of Health (P41EB025815), and the German Research Foundation (Heisenberg TH 2161/3–1, to James T. Thackeray). No other potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: Does the protocol for image acquisition in small animals affect quantitative reproducibility in cardiac imaging at multiple sites?
PERTINENT FINDINGS: The adoption of a standardized consensus acquisition protocol lowered variability in 68Ga-DOTA ECL1i (CCR2) and 68Ga-FAPI-46 (FAP) uptake values compared with a local acquisition protocol and enabled direct comparison of images acquired at a second facility. Quantitative analysis was more reproducible when conducted at a single center, supporting a core analysis model for multicenter preclinical imaging studies.
IMPLICATIONS FOR PATIENT CARE: Achieving reproducible quantitative measurements of radiotracer uptake across multiple institutions will increase the reliability of preclinical research, providing more robust biologic information, and may streamline translation to clinical applications.
Footnotes
↵* Contributed equally to this work.
Published online Jan. 4, 2024.
- © 2024 by the Society of Nuclear Medicine and Molecular Imaging.
REFERENCES
- Received for publication August 10, 2023.
- Revision received November 9, 2023.