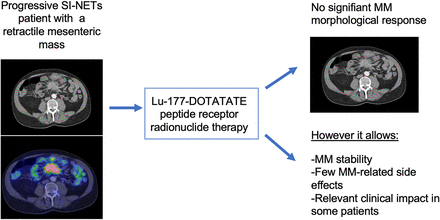
Visual Abstract
Abstract
A mesenteric mass (MM), characterized by fibrotic reaction, is present in most small-intestinal neuroendocrine tumors (SI-NETs). 177Lu-DOTATATE peptide receptor radionuclide therapy (PRRT) has shown its efficacy in patients with progressive SI-NETs. However, because of specific tissue characteristics of desmoplastic MMs, we hypothesize that these lesions may be refractory to 177Lu-DOTATATE PRRT. Methods: From the national French Groupe d’étude des Tumeurs Endocrines database, we identified patients with an advanced SI-NET and a MM (≥2 cm with a retractile aspect) of a SI-NET treated by at least 1 course of 177Lu-DOTATATE PRRT. The primary endpoint was a MM objective response rate (ORR) of less than 5%. Secondary endpoints were metabolic response, MM-related safety, and clinical response, as well as MM progression-free survival (PFS) and non-MM PFS. Results: In total, 52 patients were included. The MM ORR was 4% (n = 2), and the non-MM ORR was 8% (n = 4). No patient had a MM metabolic response, and the non-MM metabolic response rate was 12% (n = 6). Among the 26 patients with baseline MM-related symptoms, 46% had a clinical response. Four patients presented with gastrointestinal complications during PRRT. The median MM-related PFS was not reached, and the non-MM PFS was 50.3 mo (95% CI, 38.2–61.7 mo). Conclusion: This study confirms that 177Lu-DOTATATE PRRT does not lead to morphologic response on MMs (ORR < 5%). However, it allows MM stability, with few MM-related side effects, and has a relevant impact on MM-related symptoms.
Small-intestinal neuroendocrine tumors (SI-NETs) are the most common well-differentiated metastatic NETs (1). These tumors often metastasize, especially to the mesenteric lymph nodes and the liver. SI-NETs generally produce serotonin and other vasoactive substances that can lead to carcinoid syndrome (CS). The distant action of serotonin can cause fibrosis of right-sided heart valves, potentially leading to carcinoid heart disease (2,3). In addition, mesenteric lymph node metastases are often affected by fibrosis because of local serotonin secretion. In certain cases, this can lead to dense desmoplastic fibrosis, causing tethering of the bowel and mesenteric ischemia (3,4).
The efficacy of peptide receptor radionuclide therapy (PRRT) using radiolabeled somatostatin analog 177Lu-DOTATATE for progressive SI-NETs has been demonstrated (5). In the phase III NETTER-1 trial, PRRT (associated with 30 mg of long-acting release octreotide) led to a higher objective response rate (ORR, 18%), according to RECIST version 1.1; increased progression-free survival (PFS); and improved quality of life compared with a high dose of long-acting release octreotide alone (60 mg every 4 wk) in a population of patients with progressive SI-NETs (5,6). However, because of the specific tissue characteristics of desmoplastic mesenteric metastases, it was hypothesized that these lesions may be particularly refractory to PRRT, as suggested by recent studies that investigated their morphologic response (2,3) but did not set specific criteria to define a retractile mesenteric mass (MM). Furthermore, none of these studies investigated the metabolic response of MMs to PRRT, the clinical impact on MM-related symptoms, or MM-related safety. Therefore, the aim of the present retrospective study was to evaluate the ORR, according to RECIST version 1.1, on precisely defined MMs in patients with SI-NET treated with 177Lu-DOTATATE PRRT. We also evaluated the metabolic response, clinical response, and toxicity of PRRT on MMs.
MATERIALS AND METHODS
Population
Among patients treated in the 25 participant centers of the national database of the Groupe d’étude des Tumeurs Endocrines (GTE), we retrospectively identified patients with a SI-NET and a mesenteric lymph node metastasis at the time of the first dose of PRRT from January 2001 to October 2021 and managed within the national French network dedicated to NET (Endocan-RENATEN [Reseau National de Prise en Charge des Tumeurs Endocrines]-GTE). This observational study was approved on February 21, 2023, by the medical ethics research committee of the Hospices Civils de Lyon (number 22_5425). The national database of the GTE was approved on January 5, 2021, by the national data protection commission (Commission Nationale de l’Informatique et des Libertés, number 2219168). The medical records of these patients were retrospectively sought, and patients were included if they presented with a mesenteric lymph node metastasis measuring at least 2 cm in the minor axis at the time of the first PRRT use, according to RECIST version 1.1, and if they had a retractile aspect (tumor surrounded by linear bands radiating into the mesenteric fat; Fig. 1) that we defined as a MM. Other inclusion criteria were a histologically confirmed grade 1 to grade 3 SI-NET (there was no central review of the pathologic specimen, but diagnosis was made by expert pathologists from the Endocan-TENpath-GTE national network), functioning or nonfunctioning; receipt of at least 1 course of PRRT (177Lu-DOTATATE); and an available morphologic evaluation by CT or MRI performed before and after PRRT. The exclusion criteria were NETs of non–small-intestinal origin, poorly differentiated carcinomas, the absence of a MM, or a prior course of PRRT.
Baseline 68Ga-DOTATOC PET (left), CT (middle), and PET/CT (right) images of 61-y-old patient presenting with typical retractile MM surrounded by linear bands radiating into mesenteric fat (arrows) that were intensely hypermetabolic (Krenning score 4).
Parameters
We collected morphologic and metabolic imaging for central review to evaluate the potential resectability of the MM and to evaluate MM response. The resectability of the MM was based on the classification proposed by Lardière-Deguelte et al. (7) for the involvement of the superior mesenteric artery and on the study reported by Bertani et al. (8) to also include the degree of superior mesenteric venous involvement and the presence or not of mesenteric fibrosis retraction. As described by these authors, the MM was considered unresectable if the involvement of the superior mesenteric artery was classified as stage IIIup or higher (7), if proximal infiltration of the superior mesenteric vein was seen, or if there were signs of severe mesenteric fibrosis retraction (8).
The following parameters were also collected at the time of first PRRT: age, sex, NET primary location, World Health Organization classification with grading, Ki-67 index, Eastern Cooperative Oncology Group performance status (0–1 or ≥2), clinical CS, carcinoid heart disease, MM-related symptoms, number and location of metastatic sites, uptake on somatostatin receptor imaging (either 111In-octreotide scintigraphy or 68Ga-DOTATOC PET/CT), serum plasma chromogranin A (CgA) concentration (considered elevated if >2 times the upper limit of normal value at baseline), oncology treatments received before PRRT, and MM morphologic progression (according to RECIST version 1.1) before PRRT.
The characteristics and effectiveness of PRRT were collected: number of cycles, dose (full dose or reduced dose), reason for discontinuation (general health deterioration, toxicity, scheduled, or unknown), occurrence of gastrointestinal complications during PRRT (bowel obstruction, abdominal pain, motility disorders, or perforation), and corticosteroids because of the occurrence of a PRRT-related complication.
Endpoints
The primary endpoint was MM morphologic response to 177Lu-DOTATATE PRRT, according to RECIST version 1.1. A response was defined as a reduction of at least 30% in lesion diameter between the images made before and those made after PRRT. We hypothesized that the MM ORR would be less than 5% based on the study reported by Pelle et al. (2) that found no response. For 80% nominal power and an α-level of 5%, the sample size required was estimated to be 42 patients.
The secondary endpoints were MM-related safety of PRRT (occurrence of gastrointestinal complications during PRRT or need for corticosteroids), MM morphologic response in comparison to other metastatic sites (liver, adenopathy, peritoneum, etc.) for the same patient (according to RECIST version 1.1), metabolic response on MMs and on other metastatic sites, clinical response on MM-related symptoms, CS clinical response, CgA laboratory response, MM-related and non–MM-related PFS, overall survival since the first dose of PRRT, long-term clinical MM outcome (symptom response and complications), and cause of death (MM-related, NET-related but non–MM-related, non–NET-related, or unknown).
The metabolic response was defined as a decrease of at least 1 point on the Krenning scale, for 111In-octreotide scintigraphy, or according to European Organization for Research and Treatment of Cancer criteria, for 68Ga-DOTATOC PET/CT (9), on imaging before versus after PRRT. The clinical MM response was determined in patients with MM-related symptoms because of a MM at baseline and was defined as complete (no symptoms), partial (if ≥50% of baseline symptom improvement based on intensity on a 1–10 scale or frequency of the symptom), progression (if >50% of symptom aggravation based on intensity or frequency of the symptom), or stability (in case of neither response nor progression). The CS clinical response was determined in patients with baseline CS symptoms and was defined as complete, partial (if ≥50% of baseline symptom improvement based on intensity on a 1–10 scale or frequency of the symptom), progression (if >50% of symptom aggravation based on intensity or frequency of the symptom), or stability (in case of neither response nor progression). CgA laboratory response was defined as a decrease of at least 50% of the CgA level in patients with elevated baseline CgA.
Long-term gastrointestinal complications and long-term clinical MM response were both defined at the time of last news, at least 6 mo after the last PRRT dose. We also estimated the median follow-up time between the last dose of PRRT and the last news. Images were reviewed by an independent observer, who reassessed the morphologic and metabolic responses on MM and non-MM metastatic sites.
Statistical Analysis
Categoric data are expressed as number and percentage, and continuous variables are expressed as median and range. PFS was calculated from the first dose of PRRT to the date of first morphologic progression or death. Overall survival was calculated from the first dose of PRRT to the date of death or last follow-up. Survival curves for PFS and overall survival were estimated using the Kaplan–Meier method. All statistical analyses were performed using SPSS version 17.0 (IBM).
RESULTS
Patient Characteristics
Among the 25 participant centers of the GTE database, 8 centers agreed to include patients. In total, 52 patients were included; 46% were women, and the median age was 70 y (range, 47–89 y). Ileum was the primary SI-NET site in most patients (73%). SI-NETs were grade 1 (48%) or grade 2 (42%), and median Ki-67 was 2.5%. There were no grade 3 SI-NETs, but 5 patients had SI-NETs of an unknown grade. Most patients (62%) had at least 2 other metastatic sites. These metastases were mainly located in the liver (87%), distant lymph nodes (37%), peritoneum (33%), or bone (27%; Table 1). In total, 29 patients were classified as stage IIIup (n = 15) or stage IV (n = 14), according to the Lardière-Deguelte et al. (7) classification; therefore, the MM was considered unresectable. According to Bertani et al. (8), 27 patients had proximal superior mesenteric venous involvement and 18 patients had severe mesenteric fibrosis. In total, 32 (65%) patients had an unresectable MM at the time of PRRT (missing data, n = 3; Supplemental Table 1 [supplemental materials are available at http://jnm.snmjournals.org]).
Patient Characteristics at Time of First PRRT Dose, n = 52
Almost half (46%) of patients had previously undergone primary tumor resection, and 31% of patients had previously undergone liver embolization. Concerning systemic therapies, 90% of patients received prior somatostatin analogs and 19% of patients received prior everolimus (Table 1). Of the 24 patients who underwent resection of the primary tumor without resection of the MM, 14 (64%) patients had at least 1 nonresectability criterion. The other 8 patients had a potentially resectable MM, but all had synchronous metastases and all but 1 patient presented with minimal mesenteric fibrosis or peripheral superior mesenteric venous infiltration; therefore, no reoperation was performed (Supplemental Table 1). Almost all patients had an Eastern Cooperative Oncology Group performance status of 0–1 (96%) before PRRT, and 75% of patients presented with a clinical CS. Half of them had MM-related symptoms; the most frequent symptoms were mainly abdominal pain (38%), diarrhea (27%), occlusive syndrome (15%), or mesenteric ischemia symptoms (10%). All but 1 patient had a MM Krenning score of 3 (8%) or 4 (90%) at baseline somatostatin receptor imaging. More than a third (38%) of patients presented with a morphologic progression on the MM, according to RECIST version 1.1, at the time of the first PRRT dose (Table 1).
Effectiveness and Safety of PRRT
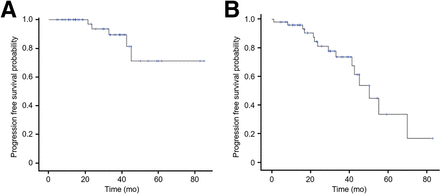
Imaging (CT or MRI) was done at a mean of 2.0 mo (range, 1 d to 7 mo) before PRRT and a mean of 4.6 mo (range, 2–11.7 mo) after PRRT. Images and both morphologic and metabolic evaluations were centrally reviewed for 43 (83%) patients, and no difference was found with the first reading. With regard to the primary endpoint, the MM ORR was 4%, and no patient had a metabolic response. The non-MM ORR was 8%, and the non-MM metabolic response rate was 12%. Among the 26 patients with baseline MM-related symptoms, 12 (46%) patients had clinical response (complete in 3 patients and partial in 9 patients). Among the 39 patients presenting with a CS, 18 (46%) patients had a complete or partial response (Table 2). Only 5 (10%) patients presented with MM RECIST progression on a median follow-up of 30.2 mo. The median MM-related PFS was not reached (Fig. 2A; Table 2) and was 50.3 mo (95% CI, 38.2–61.7 mo) on non–MM-related PFS (Fig. 2B; Table 2). The median overall survival was 55.0 mo (95% CI, 39.5–80.8 mo; Table 2). Of the patients who showed MM-related symptoms before PRRT, 42% presented with long-term persistence of partial or complete clinical response.
Effectiveness of 177Lu-DOTATATE PRRT, n = 52
Patients’ estimated MM-related PFS (A) and non–MM-related PFS (B) since first dose of PRRT.
Most patients (83%) received 4 cycles of 177Lu-DOTATATE. For those who discontinued PRRT prematurely, the main reason was the occurrence of toxicity (8% of the total population): cholestasis (n = 1), cytopenia (n = 1), and renal insufficiency (n = 2; Table 3). Four patients presented with gastrointestinal complications during PRRT: bowel obstruction and abdominal pain (n = 1), abdominal pain only (n = 1), diarrhea because of mesenteric ischemia (n = 1), or vomiting (n = 1). Two received a short course of corticosteroids (Table 3). In 6 (12%) patients, long-term gastrointestinal complications occurred (bowel obstruction, abdominal pain, constipation, perforation, or mesenteric ischemia). Two (4%) patients died from MM-related complications, which resulted from a digestive perforation for 1 of the patients (Table 3). Among the 9 patients presenting with early or late complications, 2 patients were theoretically resectable on the MM. The 2 patients who died from MM-related complications were clearly unresectable (stage IV superior mesenteric artery involvement, proximal superior mesenteric venous infiltration, and severe mesenteric fibrosis; Supplemental Table 1).
Safety of 177Lu-DOTATATE PRRT, n = 52
DISCUSSION
The present study confirms that MM does not respond morphologically to PRRT, which is concordant with the suggestions of Pelle et al. (2), who reported 0 of 21 (0%) ORR on mesenteric metastasis, and Blažević et al. (3), who reported 5 of 132 (4%) ORR on mesenteric metastasis. The strength of the present study is that it is a multicenter, national study with a retrospective central review of most morphologic and metabolic imaging that pays specific attention to MM outcomes during and after PRRT.
Although the MM ORR was low, only a few patients presented with radiologic MM progression during the follow-up, whereas more than a third of them had progressive MM disease before initiating PRRT; furthermore, the median MM-related PFS was longer than the median non–MM-related PFS. Altogether, this suggests that PRRT could lead to long-term stabilization of the MM. However, this may also be explained by the relatively static growth behavior of mesenteric metastases, as suggested by Blažević et al. (3), who found a mesenteric metastasis progression rate close to that reported herein (13.5%). The authors also suggested potential underestimation of disease progression and PRRT response on MMs resulting from the exclusive use of RECIST in this context (3). Concerning clinical effectiveness, PRRT significantly affected symptomatic patients. For instance, there was improvement of clinical CS in almost half of the patients, which is concordant with the literature: Zandee et al. (10) reported improvement of CS symptoms in at least 50% of patients with NET treated with PRRT. It is also concordant with the results of the NETTER-1 study, which demonstrate significant improvement in the quality of life of patients presenting with a NET treated with PRRT compared with results from high-dose octreotide, partly related to CS improvement (6). We also noted clinical improvement in almost half of patients presenting with baseline MM-related clinical symptoms, which reinforces the clinical value of PRRT. This is all the more important because almost half of baseline MM-related symptomatic patients presented with persistence of partial or complete clinical response more than 6 mo after the end of PRRT, suggesting a possible long-term clinical impact of PRRT. However, because 9 patients presented with early or late gastrointestinal complications and 2 patients died from MM-related complications, removal of the MM (when resectable) before PRRT should be discussed in a multidisciplinary tumor board dedicated to NETs.
The present study also indicates that PRRT can be used in patients presenting with baseline MM-related symptoms, because none of the patients in the study presented with progression of symptoms under PRRT. Moreover, relatively few gastrointestinal complications were observed during PRRT in the total population, and only 1 patient presented with bowel obstruction. There are concerns with regard to the potential occurrence of such complications, given Strosberg et al. (11) reported that PRRT can lead to bowel obstruction in patients with a mesenteric metastasis by inducing inflammation. However, Laskaratos et al. (12) suggested that there is no relationship between the severity of mesenteric fibrosis and the onset of an obstructive symptomatology. It was also suggested by Strosberg et al. (11) that a short course of corticosteroids during PRRT could potentially prevent the occurrence of gastrointestinal complications. In the present study, the patient who experienced bowel obstruction did not receive a preventive course of corticosteroids, possibly overestimating the occurrence of gastrointestinal complications if a preventive course of corticosteroids to prevent these symptoms becomes routine practice. Still, a prospective randomized study would be helpful to determine whether such prophylactic treatment would be useful in avoiding locoregional complications of PRRT without altering its efficacy. Moreover, we noted few occurrences of long-term gastrointestinal complications, which provides reassurance on the feasibility of PRRT.
An interesting aside is the finding herein that the non-MM ORR was twice as high as the MM ORR. However, this rate remains lower than that reported in the literature for patients without a mesenteric metastasis; for instance, in the NETTER-1 study, ORR was 18% (4), whereas the non-MM ORR reported by Blažević et al. (3) was closer (13%) to that found herein. This difference may be explained, at least partly, by differences in the rates of G1 SI-NET, which were higher in the NETTER-1 study (72% vs. 48% herein) (4) and closer in the study reported by Blažević et al. (52% vs. 48% herein) (3). We could also hypothesize that SI-NETs with MMs have a different carcinogenesis from NETs without MMs that is associated with a lower ORR on the non-MM metastatic sites; this has to be confirmed in prospective studies comparing patients treated by PRRT with versus without a MM.
The limitation of this study, given its retrospective nature based on patient medical records, is that the data may not be accurate among the different centers; a prospective study would be interesting to reinforce the results. In addition, 5-hydroxyindoleacetic acid was not collected; therefore, it was not possible to evaluate the biologic response on CS under PRRT in correlation with the clinical, morphologic, and metabolic responses.
CONCLUSION
This study confirms that 177Lu-DOTATATE PRRT does not lead to morphologic response on MMs (ORR < 5%). However, it allows MM stability, with few MM-related side effects, and has a relevant clinical impact on MM-related symptoms.
DISCLOSURE
This study did not receive specific grants from funding agencies in the public, commercial, or not-for-profit sectors; however, patients were identified from the French national database of the GTE, which is partially supported by IPSEN-pharma. Louis De Mestier is supported by AAA/Novartis, Esteve, IPSEN-pharma, and Sirtex; Magalie Haissaguerre is supported by Novartis, AAA, and IPSEN-pharma; Pauline Afchain is supported by IPSEN-pharma and Novartis; Julien Hadoux is supported by Lilly, AAA, IPSEN-pharma, Roche, Pharma Mar, EISAI, and Novartis; Thierry Lecomte is supported by IPSEN-pharma, AAA, and Novartis; and Thomas Walter is supported by Novartis, IPSEN-pharma, Keocyt, and Sirtex. No other potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: Is it relevant to propose 177Lu-DOTATATE PRRT to patients with progressive SI-NETs and a retractile MM?
PERTINENT FINDINGS: In a study involving 52 patients, our data confirm that 177Lu-DOTATATE PRRT does not lead to morphologic response on MMs (ORR < 5%); however, it allows MM stability, with few MM-related side effects, and a relevant clinical impact on MM-related symptoms in some patients.
IMPLICATIONS FOR PATIENT CARE: There was improvement of clinical CS in almost half of the patients and clinical improvement in almost half of patients presenting with baseline MM-related clinical symptoms, which reinforces the clinical value of PRRT. This is all the more important because almost half of baseline MM-related symptomatic patients presented with persistence of partial or complete clinical response more than 6 mo after the end of PRRT, suggesting a possible long-term clinical impact of PRRT.
ACKNOWLEDGMENTS
We thank the patients and their association (APTED) for their participation. We also thank the French organization for neuroendocrine neoplasm management, the Endocan-RENATEN clinical network (constructed and supported by the GTE), and Philip Robinson (DRS, Hospices Civils de Lyon) for his help in article preparation.
Footnotes
Published online Jan. 11, 2024.
- © 2024 by the Society of Nuclear Medicine and Molecular Imaging.
REFERENCES
- Received for publication May 25, 2023.
- Revision received October 25, 2023.