Visual Abstract
Abstract
Accurate diagnosis and staging are crucial for selecting treatment for patients with pancreatic ductal adenocarcinoma (PDAC). The desmoplastic responses associated with PDAC are often characterized by hypometabolism. Here, we investigated 18F-fibroblast activation protein inhibitor (FAPI)–04 PET/CT in evaluation of PDAC and compared the findings with those obtained using 18F-FDG. Methods: Sixty-two PDAC patients underwent 18F-FAPI-04 PET/CT and 18F-FDG PET/CT. Identification of primary lesions, lymph node (LN) metastasis, and distant metastasis (DM) by these methods was evaluated, and TNM staging was performed. Correlation between SUVmax of the primary lesion and treatment response was explored in patients who received systemic therapy. Results: 18F-FAPI-04 PET/CT identified all patients with PDAC; 18F-FDG PET/CT missed 1 patient. Tracer uptake was higher in 18F-FAPI-04 PET/CT than in 18F-FDG PET/CT in primary tumors (10.63 vs. 2.87, P < 0.0001), LN metastasis (2.90 vs. 1.43, P < 0.0001), and DM (liver, 6.11 vs. 3.10, P = 0.002; peritoneal, 4.70 vs. 2.08, P = 0.015). The methods showed no significant difference in the T staging category, but the N and M values were significantly higher for 18F-FAPI-04 PET/CT than for 18F-FDG PET/CT (P = 0.002 and 0.008, respectively). Thus, 14 patients were upgraded, and only 1 patient was downgraded, by 18F-FAPI-04 PET/CT compared with 18F-FDG PET/CT. A high SUVmax of the primary tumor did not correlate with treatment response for either 18F-FAPI-04 or 18F-FDG. Conclusion: 18F-FAPI-04 PET/CT performed better than 18F-FDG PET/CT in identification of primary tumors, LN metastasis, and DM and in TNM staging of PDAC.
Pancreatic ductal adenocarcinoma (PDAC) is one of the most lethal malignancies (1). Accurate diagnosis and initial staging are crucial for optimal treatment selection. Imaging techniques, including CT and MRI, are the most frequently used methods for tumor detection, staging, treatment response evaluation, and tumor surveillance (2,3). CT scans, which offer good resolution and wide anatomic coverage, are routinely used for tumor staging and assessment of resectability. Both local and distant diseases can be assessed in a single session (4). However, the detection of micrometastases with CT scans remains a major challenge. MRI has proved to be outstanding for detection of small lesions, including identification of local pancreatic tumors and screening for hepatic or peritoneal micrometastases. However, screening-range limitations restrict the application of MRI in the detection of distant metastases (DMs) (5).
PET/CT is a hybrid imaging technique with wide anatomic coverage that allows the depiction of all possible small metastases throughout the body. 18F-FDG is the most widely used radiotracer for PET/CT. Although hypermetabolic tumors are known to demonstrate particularly high 18F-FDG uptake, the desmoplastic reaction associated with PDAC usually shows hypometabolic characteristics, which is a well-known limitation of 18F-FDG PET/CT in PDAC diagnosis and staging (6–8).
The tumor cells in PDACs exist within a dense stroma, which is composed of an extracellular matrix, vasculature, and cancer-associated fibroblasts (9). Fibroblast activation protein (FAP) is a membrane protease that is highly expressed on the surface of cancer-associated fibroblasts (10,11). Therefore, a radioactively labeled FAP inhibitor (FAPI) is a promising PET tracer in PDAC (12,13). Moreover, PDAC is expected to show intensive uptake of 68Ga-conjugated FAPI (68Ga-FAPI). The clinical value of 68Ga-FAPI for PDAC has been preliminarily investigated, and the studies have shown promising results (14,15).
Nevertheless, storage and long-distance transit of 68Ga are difficult because of its relatively short half-life. In addition, the availability of 68Ga-labeled tracers from 68Ge/68Ga generators is limited. In contrast, 18F is the most widely used radionuclide in PET; therefore, it can be easily produced in larger doses and delivered over longer distances at a relatively lower cost than 68Ga. Thus, 18F-labeled FAPI-targeting tracers are strongly desired in clinical practice (16). However, the advantages of 18F-AlF-NOTA-FAPI-04 (18F-FAPI-04) over 18F-FDG have not yet been systematically evaluated in PDAC. Our purpose was to explore the potential efficacy of 18F-FAPI-04 PET/CT for PDAC tumor staging and compare the results with those obtained using 18F-FDG PET/CT.
MATERIALS AND METHODS
Enrollment and Treatment
Sixty-two patients with PDAC were enrolled prospectively between August 2021 and February 2023 at the First Affiliated Hospital, School of Medicine, Zhejiang University. The hospital’s ethics committee approved this study (NCT05884463; ClinicalTrials.gov), and all patients gave written informed consent. For comparative analyses, both 18F-FAPI-04 PET/CT and 18F-FDG PET/CT were performed at enrollment. The inclusion criteria were as follows: patients who were suspected to have PDAC by radiologic imaging; patients who had scheduled paired 18F-FAPI-04 PET/CT and 18F-FDG PET/CT for metastasis screening, recurrence confirmation, or tumor staging; and patients who were willing to participate in clinical trials and who signed an informed-consent form. The exclusion criteria were as follows: patients who were not pathologically diagnosed as PDAC, pregnant patients, and patients with the inability or unwillingness of the research participant, parent, or legal representative to provide written informed consent. After systemic treatment, surgical treatment was performed if the patients met the criteria for a conversion operation. The decision to complete preoperative PET/CT was based on the patient’s willingness. The treatment response was evaluated bimonthly according to RECIST version 1.1. Final clinical staging was conducted by our tumor board and based on clinical, pathologic, and all imaging data.
Radiopharmaceuticals
18F-FAPI-04 was prepared as described previously (17,18). The NOTA-FAPI-04 precursor was purchased from Beijing PET Technology Co. Ltd. 18F was produced from a medical cyclotron (Siemens Medical Solutions). The synthesis of 18F-FAPI-04 was performed in an AllInOne synthesis module (Trasis). The final product was reconstituted in saline and passed through a 0.22-μm syringe filter (Pall Corp.). The radiochemical purity of 18F-FAPI-04 was analyzed by radio–high-performance liquid chromatography (1200 series; Agilent) and was more than 95%. 18F-FDG was synthesized automatically and routinely in a 18F-FDG synthesizer module (FDG4 Explora; Siemens) and was purified to radiochemical purity of more than 95% before clinical use.
PET/CT Imaging
PET/CT imaging with both 18F-FAPI-04 and 18F-FDG was performed on a PET/CT scanner (Biograph version 600; Siemens Healthineers). All images were acquired from top of skull to mid thigh 60–90 min after intravenous administration of 18F-FAPI-04 or 18F-FDG at a dose of 3.7–4.44 MBq/kg (0.1–0.12 mCi/kg). Fasting and normal blood glucose levels were obtained for 18F-FDG PET/CT. 18F-FAPI-04 PET/CT and 18F-FDG PET/CT were performed within 2 wk, and both were conducted before treatment. The PET scan was performed with 3 min/frame three-dimensional acquisition. The CT parameters were 120 kV, 160 mA, pitch of 1.3, slice thickness of 2.5 mm, and rotation time of 0.5 s, and these were used to conduct PET attenuation correction. PET images were reconstructed using a Siemens workstation (syngo.via Client 4.1) with TrueX plus time of flight (UltraHD PET [Siemens]; 10 iterations, 5 subsets, gaussian filter with full width at half maximum of 4 mm, 440 × 440 matrix).
PET/CT Image Analysis
Two nuclear medicine physicians, both of whom have more than 10 y of experience in nuclear oncology, independently analyzed all images using a MedExsystem nuclear medical information system (MedEx Technology Limited Corp.), and discordant results were resolved by consensus. Image interpretation included visual analysis and quantitative assessments. Focal 18F-FAPI-04 or 18F-FDG accumulations showing activity higher than the background, except for physiologic uptake, were considered potential positive lesions. The uptake of 18F-FAPI-04 or 18F-FDG in primary tumors and metastatic lesions was semiquantified by SUVmax. To ensure that SUVmax was relatively comparable, the tumor-to-background (T/B) ratio was performed according to the following formula: T/B ratio = tumor SUVmax/background SUVmean. Average SUVmean of the liver was set as the background to SUVmax of the local tumor. Background SUVmean of hepatic or bone metastasis was average SUVmean of normal liver tissue or bone tissue, respectively. For lymph node (LN), pleural, and peritoneal lesions, background SUVmean was set as average SUVmean of the descending aorta. Average background SUVmean was calculated for 3 random regions. If there were fewer than 5 lesions in a single organ, all lesions were quantitatively assessed. Otherwise, the 5 lesions with the highest activity were quantitatively evaluated.
Statistical Analysis
Continuous variables were expressed as mean ± SD, whereas categoric variables were expressed as frequency and proportion. 18F-FAPI-04 and 18F-FDG uptake were compared using the paired t test. The McNemar–Bowker test was used to assess significant differences between 18F-FAPI-04 and 18F-FDG PET/CT for TNM staging. All statistical analyses were conducted using SPSS (version 18.0; IBM).
RESULTS
Participant Characteristics
All patients were pathologically diagnosed as showing PDAC by biopsy or surgery. Fifty-eight patients were newly diagnosed and treatment-naïve, whereas the other 4 patients underwent PET/CT for restaging after initial treatment. Our cohort consisted of 43 men and 19 women, with a median age of 63 y. Finally, 54 patients received further treatment at our institution, including surgery treatment (n = 4) and systemic treatment (n = 50). In addition, 48 patients who received systemic treatment underwent radiologic response evaluation; these patients were included to investigate the value of the 2 tracers in treatment response prediction. More details about the patients’ concurrent symptoms, comorbidities, tumor location, carbohydrate antigen 19-9 values, and other pertinent data are recorded in Supplemental Table 1 (supplemental materials are available at http://jnm.snmjournals.org).
Adverse Events
18F-FAPI-04 and 18F-FDG were tolerated by all participants without physical discomfort or adverse effects.
Diagnostic Performance of 18F-FAPI-04 and 18F-FDG in Primary Tumors
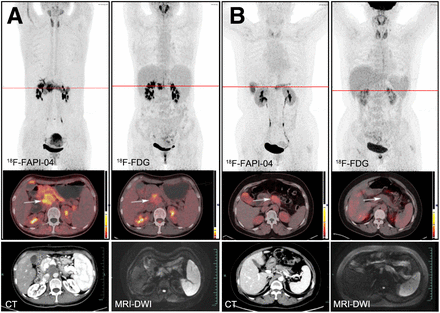
18F-FDG PET/CT showed a sensitivity of 98.4% (61/62 patients) for identification of primary tumors, whereas 18F-FAPI-04 PET/CT identified all local lesions (62/62 patients). 18F-FAPI-04 SUVmax was almost 2 times greater than 18F-FDG SUVmax, increasing from a mean of 8.00 (range, 3.70–55.20) to 15.65 (range, 3.70–34.50) in the semiquantitative parametric analysis (Table 1) and showing that the uptake of 18F-FAPI-04 in primary tumors was significantly greater than that of 18F-FDG (P < 0.0001). The difference of T/B ratio in uptake between 18F-FAPI-04 and 18F-FDG was more pronounced (10.63 vs. 2.87, P < 0.0001). The typical PET/CT images obtained with the 2 tracers and the corresponding CT/MR images are shown in Figure 1.
Comparison of 18F-FDG and 18F-FAPI Uptake in Lesions
Typical PET (top), PET/CT (middle), and CT and MR (bottom) images of primary tumor obtained using 2 tracers in representative patients (A and B). Tumor is marked by arrows. DWI = diffusion-weighted imaging.
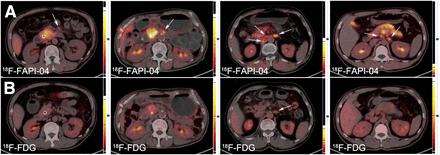
Diagnostic Performance of 18F-FAPI-04 and 18F-FDG for LN Assessments
In total, 44 patients showed large LN shadowing with high metabolism after performing PET/CT. Among these, 40 patients showed abnormal LN findings on 18F-FDG PET/CT, whereas the remaining 4 patients showed suggestive findings on 18F-FAPI-04 PET/CT alone (Table 1). 18F-FAPI-04 showed an obvious advantage over 18F-FDG in terms of the number of positive LNs identified (203 vs. 151). In the semiquantitative study, median SUVmax and maximum SUVmax for 18F-FAPI-04 uptake were 3.56 and 10.32, respectively, which were higher than the values for 18F-FDG (median SUVmax, 2.30; maximum SUVmax, 5.92), with a P value of less than 0.0001. The difference in uptake between 18F-FAPI-04 and 18F-FDG was more pronounced in the T/B ratio (2.90 vs. 1.43, P < 0.0001). The 2 examination approaches showed a substantial difference for the identification of LN metastases (Fig. 2).
Typical LN PET/CT images obtained with 18F-FAPI-04 (A) and 18F-FDG (B) from 4 patients. Lesion is marked by arrows.
Diagnostic Performance of 18F-FAPI-04 and 18F-FDG for DM
The data for the number of positive hepatic, peritoneal, bone, and pleural metastases and the semiquantitative parameters of 18F-FAPI-04 PET/CT and 18F-FDG PET/CT are presented in Table 1. 18F-FDG and 18F-FAPI-04 confirmed hepatic metastasis in 5 and 12 patients, respectively, implying that 18F-FAPI-04 surpassed 18F-FDG in the detection of hepatic lesions. SUVmax in hepatic metastases was slightly higher for 18F-FAPI-04 than for 18F-FDG (7.04 vs. 6.10), but the difference was not significant (P = 0.388). To exclude background effects, the T/B ratio of 18F-FAPI-04 was higher than that of 18F-FDG (6.11 vs. 3.10, P = 0.002). Altogether, 18F-FAPI-04 PET/CT showed better sensitivity and accuracy than 18F-FDG PET/CT for detection of hepatic metastases. The images of representative cases are presented in Figure 3. Similar results were obtained for patients with peritoneal metastasis. Although the sample size of patients with bone or pleural lesions was limited, 18F-FAPI-04 PET/CT demonstrated higher detection rates of these lesions than did 18F-FDG PET/CT (Fig. 4).
Typical PET (top), PET/CT (middle), and CT and MR (bottom) images of hepatic metastases obtained using 2 tracers in 2 patients (A and B). Lesion is marked by arrows. DWI = diffusion-weighted imaging.
Typical PET (top) and PET/CT (middle and bottom) images showing pleural (A) and peritoneal (B) metastasis obtained with 2 tracers. Axial PET/CT images correspond to red lines in coronal PET images. Lesion is marked by arrows.
TNM Staging
Sixty-two patients were staged according to the eighth edition American Joint Committee on Cancer tumor staging criteria (Supplemental Table 2). The distribution of T staging was similar between the 2 tracers. Assessment of vascular involvement based on enhanced CT was more accurate than that based on PET/CT. Therefore, the T4 staging proportion based on CT/MRI (58.1%) was significantly greater than that based on PET/CT.
N staging was more variable between 18F-FDG and 18F-FAPI-04. Four patients without LN metastases, according to 18F-FDG, were categorized as N1 by 18F-FAPI-04, and 11 patients who were categorized as N1 according to 18F-FDG were categorized as N2 by 18F-FAPI-04. Moreover, preoperative 18F-FAPI-04 PET/CT was performed in 13 patients. Pathologic examination confirmed 290 LNs. Of these, 23 positive LNs were confirmed in 6 patients. LN involvement included 18 true-positive, 26 false-positive, 241 true-negative, and 5 false-negative findings with 18F-FAPI-04 PET/CT. The sensitivity, specificity, and accuracy for the diagnosis of LN metastasis were 78.3%, 90.3%, and 89.3%, respectively (Supplemental Table 3).
18F-FDG PET/CT revealed DM in 17 patients, whereas 18F-FAPI-04 PET/CT showed DM in 24 patients. 18F-FAPI-04 PET/CT upgraded the M stage in 7 patients. Five of them were confirmed to have hepatic metastasis by 18F-FAPI-04 PET/CT, whereas the remaining 2 patients were found to have peritoneal metastases and bone metastases.
Figure 5 illustrates how, in comparison with 18F-FDG PET/CT, 18F-FAPI-04 PET/CT upgraded the staging of 14 patients: 1 from Ia to IIb, 1 from Ib to IIa, 1 from Ib to IIb, 2 from IIa to IV, 4 from IIb to III, 4 from IIb to IV, and 1 from III to IV. However, only 1 patient was downstaged from III to IIb after 18F-FAPI-04 PET/CT (Supplemental Tables 4 and 5).
Staging based on CT/MRI, 18F-FAPI-04 PET/CT, and 18F-FDG PET. Shown are number of patients in T, N, and M categories (A); prognostic stage groups based on CT/MRI, 18F-FAPI-04 PET/CT, and 18F-FDG PET/CT (B); and differences in prognostic staging of patients between 2 tracers (C).
Treatment Response Evaluation
Forty-eight patients received systemic treatment, and the best treatment response was recorded. The correlations between SUVmax or T/B ratio and response were analyzed (Fig. 6). Median SUVmax and median T/B ratio values of 18F-FDG and 18F-FAPI-04, respectively, were identified as the cutoff values. Patients were divided into response group (complete and partial response) and nonresponse group (stable and progressive disease). Patients showing higher uptake of 18F-FDG (≥8.00) or 18F-FAPI (≥15.70) showed response rates similar to those of patients with lower SUVmax (18F-FDG, 25.0% vs. 21.7%, P = 0.798; 18F-FAPI, 25.0% vs. 20.8%, P = 0.786). Similarly to SUVmax, a lower 18F-FAPI-04 T/B ratio was not significantly associated with an increased response rate (29.2% vs. 16.7%, P = 0.303). Therefore, the level of uptake of 18F-FAPI-04 or 18F-FDG failed to predict the response to systemic treatment.
Relationship between SUVmax and treatment response. Shown are 18F-FDG and 18F-FAPI-04 SUVmax (A) and 18F-FDG SUVmax and 18F-FAPI-04 SUVmax T/B ratio (B) based on primary tumor and related treatment response in patients. CR+PR = complete response and partial response; ORR = objective response rate; SD+PD = stable disease and progressive disease.
DISCUSSION
Diagnosis and proper staging based on imaging assessments are essential for choosing treatment plans for tumor patients. Unfortunately, CT, MRI, and other routinely used imaging examinations frequently fall short in various aspects, especially in assessments of PDAC. Our results demonstrate that 18F-FAPI-04 PET/CT is significantly superior to 18F-FDG PET/CT in detecting both primary and metastatic lesions.
The most widely used PET tracer is 18F-FDG, which relies on functional activity to distinguish metabolically active proliferative lesions, because tumors frequently accumulate 18F-FDG (19). However, the use of 18F-FDG PET/CT for the detection and staging of suspected PDAC remains debatable (6). The sensitivity of 18F-FDG PET/CT in the initial diagnosis of PDAC ranges from 73% to 94% (20), and our study results were slightly higher than this range (∼98.4%). In contrast to 18F-FDG, the tracer 18F-FAPI-04 offers a new method for identification of malignancies (11,12). Pang et al. (12) reported that 68Ga-FAPI was more sensitive than 18F-FDG for the identification of PDAC, although their study included only 26 patients. Our study had a larger sample size: 62 PDAC patients were enrolled. In our investigation, 18F-FAPI-04 had a remarkably higher T/B ratio than that of 18F-FDG, although its identification of primary tumors was similar to that of 18F-FDG. A previous study demonstrated that 68Ga-FAPI PET/CT can be used to determine the expression of FAP and further guide 177Lu-FAPI radionuclide therapy in patients with breast cancer (21). Our study confirmed that PDAC shows high uptake of 18F-FAPI-04, which may also indirectly represent the high expression of FAP in PDAC, giving a diagnostic and clinical strategy for treatment.
LN metastasis is one of the independent factors affecting the prognosis (22). Particular importance should be placed on preoperative examination and prediction of LN status. However, 18F-FDG shows limited utility in assessing LN metastasis. In a study by Wang et al. (23), the accuracy of 18F-FDG in determining LN metastasis of PDAC in 160 patients was only 39.4%. The authors theorized that this may be related to LN size. Positive LNs often have a large number of cancer-associated fibroblasts, which can be combined with 18F-FAPI for visualization (24). In our investigation, 18F-FAPI-04 showed an obvious advantage over 18F-FDG in terms of the number of positive LNs detected and higher tracer uptake, suggesting that 18F-FAPI-04 is more sensitive than 18F-FDG in the identification of metastatic LNs. In our study, 13 patients who received tumor resection underwent 18F-FAPI-04 PET/CT preoperatively, and 290 LNs were confirmed with pathologic examination. The sensitivity, specificity, and accuracy of the diagnosis of LN metastasis based on 18F-FAPI-04 PET/CT were 78.3%, 90.3%, and 89.3%, respectively, which implies that 18F-FAPI-04 PET/CT performed well in detecting metastatic LNs. However, we did not find pathologic evidence to support the advantages of 18F-FAPI-04 PET/CT over 18F-FDG PET/CT in the assessment of LN metastasis, because preoperative paired PET/CT was not essential according to our study design.
The 18F-FDG detection findings for hepatic metastases are equally unsatisfactory (25,26). Pang et al. (12) and Deng et al. (15) have demonstrated that 68Ga-FAPI is more effective than 18F-FDG in distinguishing hepatic metastases from PDAC and gastrointestinal cancers, respectively. Similarly, hepatic metastasis was indicated by 18F-FDG alone in only 5 patients in our study. 18F-FAPI-04 and 18F-FDG had a similar SUVmax. High uptake of 18F-FDG in the liver background may cover the uptake in some micrometastases. In contrast, 18F-FAPI-04 showed better background contrast with lower uptake in the liver. Similar results were observed for peritoneal, bone, and pleural lesions. Thus, 18F-FAPI-04 upstaged 14 patients in comparison with 18F-FDG findings. Although detection of metastatic lesions by PET/CT has improved greatly, the assessment of vascular involvement based on enhanced CT is more accurate.
Some studies have already shown that the high expression of FAP on cancer-associated fibroblasts is strongly associated with aggressive tumor behavior and poor prognoses (27,28). PDAC patients with moderate or strong FAP expression experience shorter overall survival than those with negative or weak expression (29). Pancreatic tumor cells are known to exist within a dense stroma, which accounts for nearly 90% of the tumor mass. Therefore, 18F-FAPI-04 uptake is better than 18F-FDG uptake as a possible indicator of tumor prognosis. Moreover, the presence of an abundant stromal compartment may create a physical barrier to decrease microvascularity and drug delivery in the tumor, thereby reducing the sensitivity to systemic therapy. In this regard, the visualization of FAP expression using 18F-FAPI-04 seems to be a promising approach to predict the response to systemic treatment. In our study, we evaluated the correlation between 18F-FAPI-04 uptake and treatment response, but no significant difference was observed in the objective response rate in relation to differences in 18F-FAPI-04 versus 18F-FDG uptake. This may result from the limitation of the radiologic response for PDAC: it is difficult to observe obvious tumor shrinkage even in cases showing significant tumor cell regression. Because all stages of PDAC were included in our study and some patients underwent conversion surgery after treatment, we failed to analyze the correlation of SUVmax with progression survival, which is an obvious limitation. Thus, additional studies are required to validate the prognostic value of 18F-FAPI-04.
This study had some other limitations. First, we included only patients with pathologically diagnosed PDAC, and the assessment of 18F-FAPI-04 was limited to evaluating the sensitivity of this technique, with no assessments of the specificity and other indicators. Disease lesions such as those presenting in IgG4-related disease are known to show significant fibrosis, as well as the potential for high 18F-FAPI uptake (30). Furthermore, pathologic evidence to support the advantages of 18F-FAPI-04 over 18F-FDG in the assessment of LN metastasis was insufficient, because all enrolled patients underwent 18F-FAPI-04 and 18F-FDG PET/CT at diagnosis, and preoperative PET/CT was not essential according to our study design.
CONCLUSION
Our results show that 18F-FAPI-04 performed better than 18F-FDG in identifying the primary tumor, LN metastasis, and DM and for TNM staging in PDAC. In the future, 18F-FAPI-04 PET/CT may play a greater role in the actual clinical management of PDAC.
DISCLOSURE
This work was supported by the National Key Research and Development Program (2019YFC1316000 to Tingbo Liang), the National Natural Science Foundation of China (U20A20378, 81830089, and 82188102 to Tingbo Liang; 81871925 and 82071867 to Xueli Bai; 82071965 to Xinhui Su; 82071916 to Xiang Li; and 82172859 to Yiwen Chen), the Key Research and Development Program of Zhejiang Province (2019C03019 to Tingbo Liang and 2020C03117 to Xueli Bai), the Fundamental Research Funds for the Zhejiang Provincial Universities (2021XZZX031 to Xueli Bai), and Huadong Medicine Joint Funds of the Zhejiang Provincial Natural Science Foundation of China (LHDMZ22H300010 to Xinhui Su). No other potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: Is 18F-FAPI-04 PET/CT more effective than 18F-FDG PET/CT at identifying primary lesions, LN metastases, and DMs of PDAC?
PERTINENT FINDINGS: In this 62-patient prospective study, 18F-FAPI-04 PET/CT showed performance superior to that of 18F-FDG PET/CT in the detection of primary lesions and metastases of PDAC and eventually upgraded the TNM stage in 14 patients.
IMPLICATIONS FOR PATIENT CARE: 18F-FAPI-04 PET/CT is expected to assist in the detection of PDAC, offer more accurate staging, and help patients choose surgery or other treatment options.
Footnotes
- © 2024 by the Society of Nuclear Medicine and Molecular Imaging.
Immediate Open Access: Creative Commons Attribution 4.0 International License (CC BY) allows users to share and adapt with attribution, excluding materials credited to previous publications. License: https://creativecommons.org/licenses/by/4.0/. Details: http://jnm.snmjournals.org/site/misc/permission.xhtml.
REFERENCES
- Received for publication July 21, 2023.
- Revision received November 7, 2023.