Visual Abstract
Abstract
Improved imaging modalities are needed to accurately stage patients with muscle-invasive bladder cancer (MIBC) and metastatic urothelial carcinoma. Imaging with small-molecule ligands or inhibitors of fibroblast activation protein (FAP) is a promising modality that has demonstrated initial efficacy across a broad range of tumors. We present our experience with the novel FAP-peptide binder 68Ga-FAP-2286 in patients with MIBC. Methods: Patients with histopathologically confirmed bladder cancer who had either localized disease at diagnosis (localized cohort, n = 13) or known metastatic disease (metastatic cohort, n = 8) were imaged with 68Ga-FAP-2286 PET as part of a clinical trial (NCT04621435). The SUVmax of 68Ga-FAP-2286 PET–positive lesions and lesion size were documented. In patients who had available 18F-FDG PET performed within 45 d of 68Ga-FAP-2286 PET (n = 5), uptake on the 2 scans was compared. When there was a discrepancy between imaging modalities on retrospective review, biopsy of suggestive lesions was performed as the standard of care. Results: In the metastatic and localized cohorts, 36 and 18 68Ga-FAP-2286–avid lesions, respectively, were identified across multiple anatomic locations, including lymph nodes, visceral metastases, and bones. Fourteen of 36 lesions in the metastatic cohort and 14 of 18 lesions in the localized cohort were lymph nodes measuring less than 1 cm. Among lesions measuring less than 0.5 cm, 0.5–1 cm, and more than 1 cm, average SUVmax was 5.2 ± 2.6, 9.6 ± 3.7, and 13.0 ± 4.3, respectively, in the metastatic cohort and 10.5 ± 5.1, 10.8 ± 5.7, and 9.9 ± 5.4, respectively, in the localized cohort. Five patients had 18F-FDG PET available for comparison. The average SUVmax for lesions avid on 68Ga-FAP-2286 PET and 18F-FDG PET was 9.9 ± 3.4 versus 4.2 ± 1.9, respectively (n = 16 lesions). For 3 patients in the localized cohort, 68Ga-FAP-2286 PET informed clinical management, including identification of both false-positive findings on 18F-FDG PET and false-negative findings on conventional CT. Conclusion: 68Ga-FAP-2286 imaging is highly sensitive in patients with urothelial cancer and is effective in identifying metastatic lesions across a variety of anatomic sites, including subcentimeter lymph nodes that would not have raised suspicion on conventional scans. This novel imaging modality may inform clinical decision-making in patients with MIBC both by refining local nodal staging and by defining metastatic disease that would otherwise be undetectable on conventional imaging.
Bladder cancer is an aggressive and potentially life-threatening disease. At diagnosis, most cases (∼75%) are identified as non–muscle-invasive (non-MIBC) which can be treated with intravesical treatments alone such as Bacillus Calmette–Guérin intravesical therapy. Although the response rate to Bacillus Calmette–Guérin therapy is high, many patients experience recurrence in the first 5 y (1). Additionally, a significant fraction (∼20%) has MIBC at diagnosis, and 5%–10% present with de novo metastatic disease (2). The standard-of-care treatment for localized MIBC is neoadjuvant cisplatin-based chemotherapy followed by radical cystectomy with curative intent (3). In those who are not candidates for cystectomy, a combination of maximal resection, chemotherapy, and localized radiation can also be used. Among several key prognostic factors, involvement of regional lymph nodes portends inferior outcomes, including decreased overall survival (4). Thus, to aid in treatment planning with either approach, as well as possible adjuvant therapy after treatment, timely and accurate nodal staging and identification of any metastatic lesions are critical.
Currently accepted imaging modalities (i.e., conventional imaging) for staging rely on size criteria or metabolic activity for accurate staging and include contrast-enhanced CT of the chest, abdomen, and pelvis; contrast-enhanced MRI of the abdomen and pelvis; and 18F-FDG PET. The criteria for identifying malignant lymph nodes in pelvic tumors are an active area of discussion and investigation. For example, on the basis of the widely used RECIST, lymph nodes can be deemed as malignant (target lesions) at initial staging if they measure more than 1.5 cm in the short axis, irrespective of tumor type or anatomic location (5). However, anatomic location and tumor type likely influence whether a node is involved by tumor. In this context, it is increasingly appreciated that pelvic lymph nodes across multiple tumor types, such as rectal cancer and MIBC, may contain malignancy despite measuring less than 1 cm (6). Accordingly, conventional scans are estimated to have approximately 50% sensitivity to accurately define nodal disease in MIBC (7). To obviate the limitations of using size-based criteria alone, 18F-FDG PET has also been evaluated for initial staging of MIBC. However, sensitivity estimates for 18F-FDG PET remain at approximately 50% (7–9). Because of physiologic 18F-FDG excretion in urine, 18F-FDG PET provides limited information about tumors within the urinary tract. Thus, there is a critical need for improved imaging modalities in patients with MIBC (7).
Fibroblast activation protein (FAP) is a transmembrane serine protease that is overexpressed on cancer-associated fibroblasts that are present in the microenvironment of diverse tumor types of epithelial, mesenchymal, and even lymphoid origin (10–12). Expression of FAP appears to be specific to cancer-associated fibroblasts, as well as a subset of fibroblasts involved in wound healing and potentially inflammatory states (11,12). Thus, FAP is an attractive pan-cancer target for both diagnostic and therapeutic applications.
Several classes of FAP-targeting molecules, including small-molecule inhibitors (FAPIs) and peptide binders, are being developed for this purpose. The FAPI family of compounds has been the best studied and has identified lesions associated with diverse epithelium-based tumors (13,14). The cyclic peptide binder FAP-2286 was recently developed to improve tumor residence time relative to FAPI (15). First-in-humans diagnostic studies of FAP-2286 have shown preliminary efficacy in staging multiple tumor types, including head-and-neck, gastric, and pancreatic cancers (16). Therapeutically, the LUMIERE trial (NCT04939610) is currently testing application of 177Lu-FAP-2286 in patients with various solid tumors, with no dose-limiting toxicities or serious adverse advents observed and a partial response noted in at least 1 enrolled patient (17).
Immunohistochemical studies have revealed high expression of FAP in urothelial carcinomas (18), with minimal expression in normal urothelium and stroma and generally high signal-to-background expression, suggesting potential diagnostic efficacy of FAP-based imaging in MIBC and metastatic urothelial carcinoma. Two initial studies showed that FAPI-46 and FAPI-04 could accurately identify bladder cancer metastases (19,20). Therefore, in this study, we investigated the ability of 68Ga-FAP-2286 to stage MIBC.
MATERIALS AND METHODS
Study Design
This is a report on a subset of patients with solid tumors prospectively enrolled in a single-arm trial (NCT04621435) evaluating the efficacy and safety of 68Ga-FAP-2286 PET from December 2020 to February 2023. This study was approved by the University of California San Francisco institutional review board, and all patients provided written informed consent. The main eligibility criteria were an age of at least 18 y, histopathologically confirmed solid tumors, and conventional imaging within 8 wk of 68Ga-FAP-2286 PET. The cohorts reported for this study include patients with metastatic, RECIST (version 1.1)-measurable disease (metastatic cohort) and patients with localized invasive bladder cancer without RECIST-measurable disease (localized cohort). Two patients with known metastatic disease before enrollment in the study were without RECIST-measurable disease at the time of imaging because of prior systemic treatment; these patients were assigned to the metastatic cohort. One patient had high-grade T1 disease with 18F-FDG PET–avid nodes clinically suggesting at least muscle-invasive disease; this patient underwent 68Ga-FAP-2286 PET despite noninvasive disease (case 2 in “Impact on Clinical Management”). The histology of the primary tumor was based on the dominant histologic pattern (e.g., >50%) of the tumor as determined by board-certified pathologists.
Imaging Protocol
All patients underwent 68Ga-FAP-2286 PET. Radiosynthesis was conducted in an iQS 68Ga fluidic labeling module and cassette (ITM Pharma Solutions GmbH). The precursor, FAP-2286, was provided by Clovis Oncology, Inc. 68Ga was eluted from a 68Ge/68Ga generator (Galliapharm; Eckert and Ziegler). Radiolabeling was performed at 120°C for 10 min. The injected activity ranged from 111 to 296 MBq (3–8 mCi), and the patients received a mean of 219.04 ± 51.8 MBq (5.92 ± 1.4 mCi). The target uptake period was 60 min (allowed range, 50–100 min), and image acquisition began at a mean of 106 ± 26 min after injection. Patients were imaged using either PET/CT (16 patients) or PET/MRI (5 patients). For PET/CT, a unenhanced diagnostic CT scan with 5-mm slice thickness was performed. For PET/MRI, abbreviated pelvic PET/MRI was followed by whole-body MRI. Whole-body PET images were acquired from pelvis to vertex. All PET images were corrected for attenuation, dead time, random events, and scatter.
Image Interpretation
All images were interpreted by a board-certified nuclear medicine physician. Uptake was considered positive if it was 1.5 times higher than blood pool activity in the mediastinum and not due to known physiologic causes. Because of physiologic excretion of the radiotracer in the bladder, lesions outside the primary bladder tumor were selected for further analysis. Up to 5 lesions per patient were included for analysis. For each lesion, the SUVmax, short-axis diameter of lymph nodes, and long-axis diameter of soft-tissue lesions were measured. In patients (n = 5) who underwent 18F-FDG PET within 45 d of 68Ga-FAP-2286 PET, paired SUVmax was measured on the 18F-FDG PET images.
Change in Management
In the localized cohort, a post hoc analysis of each patient was performed to assess for the potential impact of 68Ga-FAP-2286 PET on clinical management. Patients were categorized as those for whom 68Ga-FAP-2286 PET was concordant with conventional imaging, those for whom 68Ga-FAP-2286 PET was discordant with conventional imaging but did not alter clinical management, or those for whom 68Ga-FAP-2286 PET was discordant with conventional imaging and altered clinical management. When suggestive lesions were identified either on conventional imaging or on 68Ga-FAP-2286 PET and biopsy would influence treatment planning, biopsy was performed as the standard of care to evaluate the presence of malignancy.
Statistical Analysis
Statistical analysis was performed using GraphPad Prism software, version 9.0. For analyses comparing SUVmax across different lesion sizes within either the localized or the metastatic cohort, 1-way ANOVA with a Tukey posttest was performed. For analyses of paired SUVmax obtained from 68Ga-FAP-2286 PET versus 18F-FDG PET, a paired t test was performed.
RESULTS
Eight patients were enrolled in the metastatic cohort and 13 in the localized cohort. All had tumors with a urothelium-predominant histology (>50% urothelial) (Tables 1 and 2).
Clinical Features of Metastatic Cohort
Clinical Features of Localized Cohort
Metastatic Cohort
To assess the feasibility of 68Ga-FAP-2286 PET for identifying bladder cancer lesions as a proof of concept, we first evaluated avidity in the metastatic cohort. All 8 patients had at least 3 and at most 5 68Ga-FAP-2286–avid lesions. In total, 36 68Ga-FAP-2286–avid lesions were identified in various anatomic locations ranging from lymph nodes to visceral metastases (Fig. 1A). Notably, in addition to RECIST-measurable disease obtained on conventional imaging, 14 of 36 68Ga-FAP-2286–avid lesions measured less than 1 cm, including 12 lymph nodes, 1 lung metastasis, and 1 osseous metastasis. Among these, 5 measured less than 0.5 cm. The average SUVmax in lesions measuring less than 0.5 cm, 0.5–1 cm, and more than 1 cm was 5.2 ± 2.6, 9.6 ± 3.7, and 13.0 ± 4.3, respectively, compared with an average blood pool SUVmax of 1.4 ± 0.06. Moreover, the average SUVmax of lesions smaller than 0.5 cm was significantly lower than those larger than 1 cm (P = 0.001; Fig. 1A).
SUVmax of lesions in patients undergoing 68Ga-FAP-2286 PET. Lesions beyond bladder identified by 68Ga-FAP-2286 PET were categorized by size (<0.5, 0.5–1, and >1 cm) and are displayed for metastatic cohort (A) and localized cohort (B). Anatomic locations of lesions, including lymph nodes measuring <1 cm, are color-coded by location. Dotted line indicates blood pool background SUV averaged across all patients in cohort. Means and SDs are plotted. For metastatic cohort, range of lesion sizes was 0.3–5.5 cm; for localized cohort, range was 0.4–1.2 cm. Anatomic locations for “other” were abdominal wall (SUV, 16.8) and chest wall (SUV, 10.4) for metastatic cohort and perineum (SUV, 8.2) for localized cohort. SUVmean differed significantly between lesions measuring <0.5 cm and >1 cm (P = 0.001) in metastatic cohort. Otherwise, there was no statistically significant difference between SUVmean across different size subsets in either cohort. Of 13 patients in localized cohort, only 7 had 68Ga-FAP-2286–avid lesions.
Localized Cohort
Of the 13 patients with localized disease at initial staging, 7 had a total of 18 68Ga-FAP-2286–avid lesions (range, 1–5 lesions per patient). Almost all 68Ga-FAP-2286–avid lesions (17/18, 94%) were lymph nodes, with 1 remaining lesion a possible perineal metastasis (biopsy not performed). Similar to findings in the metastatic cohort, 68Ga-FAP-2286 PET detected subcentimeter lymph nodes (14/18 total lesions), including 4 of 18 lymph nodes measuring less than 0.5 cm. For lesions measuring less than 0.5 cm, 0.5–1.0 cm, and more than 1 cm, the average SUVmax was 10.5 ± 5.1, 10.8 ± 5.7, and 9.9 ± 5.4, respectively. The average SUVmax in lesions less than 0.5 cm did not significantly differ from that in lesions measuring 0.5–1 cm or more than 1 cm (P = 0.995 and 0.986, respectively; Fig. 1B).
Six of 7 patients with 68Ga-FAP-2286–avid lesions underwent standard-of-care biopsy of at least 1 of the lymph nodes, either as part of standard lymph node dissection during cystectomy or for biopsy of a suggestive lesion (case 1 in “Impact on Clinical Management”). Of the 7 patients, malignancy was present in at least 1 of the lymph nodes in 4 patients; in the 3 remaining patients, 1 patient underwent maximal resection, chemotherapy, and localized radiation (and therefore no pelvic lymph node sampling), and 2 patients had ypT0N0 pathology in the postcystectomy specimens. The patients who were to receive neoadjuvant therapy underwent 68Ga-FAP-2286 PET before that therapy.
68Ga-FAP-2286 PET Versus 18F-FDG PET
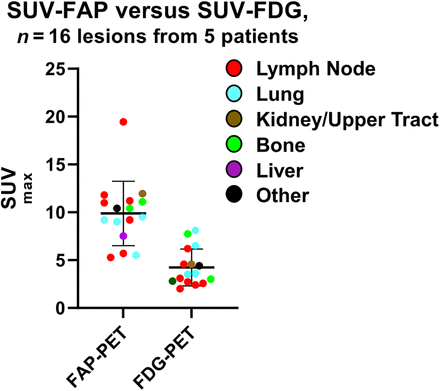
Across both cohorts, 5 total patients (3 from the metastatic cohort and 2 from the localized cohort) had paired 18F-FDG PET available for comparison. In these patients, all 16 68Ga-FAP-2286–avid lesions were also 18F-FDG–avid. The average SUVmax for 68Ga-FAP-2286–avid lesions was 9.9 ± 3.4, which was significantly greater than the average SUVmax of 18F-FDG PET–avid lesions, 4.2 ± 1.9 (P < 0.0001) (Fig. 2). Of 16 total lesions, 1 (located in the lung) had higher uptake on 18F-FDG PET, whereas the remainder had higher uptake on 68Ga-FAP-2286 PET. In 1 case, a patient had indeterminate pelvic nodes on 18F-FDG PET that were negative on 68Ga-FAP-2286 PET; at the time of surgery, the nodes were negative for malignancy (Supplemental Fig. 1; supplemental materials are available at http://jnm.snmjournals.org).
SUVmax of lesions from 68Ga-FAP-2286 vs. 18F-FDG PET. SUVs of lesions beyond bladder were identified by 68Ga-FAP-2286 PET or paired 18F-FDG PET in 5 patients (3 from metastatic cohort; 2 from localized cohort). Means and SDs are plotted. SUVmax differed significantly between 68Ga-FAP-2286 PET and 18F-FDG PET (P < 0.0001).
Impact on Clinical Management
We performed a post hoc analysis to assess the concordance of 68Ga-FAP-2286 PET with conventional imaging in the localized cohort and ascertain potential clinical implications. Among the 13 patients, 68Ga-FAP-2286 PET was concordant with conventional imaging in 5, nonconcordant with conventional imaging but did not change management in 5, and nonconcordant with conventional imaging but did change clinical management in 3. We describe these 3 cases below.
Case 1
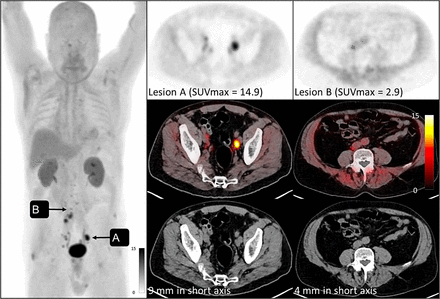
A 76-y-old man presented with clinically localized disease based on conventional imaging (Fig. 3). CT did not demonstrate enlarged lymph nodes, and the patient was originally scheduled for a radical cystectomy. 68Ga-FAP-2286 PET demonstrated numerous avid nodes (SUVmax and size of detected lymph nodes: 16.1 and 0.6 cm, respectively) extending to the left supraclavicular region, suggesting metastatic disease. Biopsy of the left supraclavicular node confirmed the presence of metastatic urothelial cancer. Radical cystectomy was deferred, and the patient was instead referred for systemic therapy to treat metastatic disease.
68Ga-FAP-2286 PET (top and maximal-intensity projection at left), PET/CT (middle), and CT (bottom) in 76-y-old man with MIBC. Imaging revealed metastatic disease in subcentimeter lymph node not detectable by conventional CT, including disease in supraclavicular node (arrow), which was biopsy-confirmed to contain bladder cancer.
Case 2
A 28-y-old man presented with a history of high-volume, rapidly recurrent, high-grade T1 bladder cancer (high-risk non-MIBC) with 6- to 7-mm pelvic lymph nodes identified on CT urography but with otherwise presumed organ-confined disease (Fig. 4). He had undergone intravesical Bacillus Calmette–Guérin treatment 2 mo before the scans. 18F-FDG PET demonstrated uptake in these nodes, raising suspicion of muscle-invasive disease and nodal metastasis. 68Ga-FAP-2286 PET, however, showed no uptake in these lymph nodes, raising the possibility that this uptake on 18F-FDG PET was false-positive. The patient underwent excisional lymph node sampling (robot-assisted) because percutaneous biopsy was deemed unsafe. The sampling demonstrated that none of the 13 evaluated nodes were positive for tumor, including the lymph nodes in question. It is likely that his prior extensive transurethral resections and subsequent intravesical Bacillus Calmette–Guérin treatment may have led to benign inflammation of these nodes, thereby leading to the false-positive findings on 18F-FDG PET.
68Ga-FAP-2286 and 18F-FDG PET (left), PET/CT (middle), and CT (right) in 28-y-old man with non-MIBC. 18F-FDG PET showed positive nodes concerning for metastatic disease, whereas 68Ga-FAP-2286 PET showed no uptake. Lymph node dissection confirmed absence of cancer in lymph nodes. Avidity seen on 68Ga-FAP-2286 PET is from excretion of tracer into left ureter.
Case 3
A 78-y-old man presented with MIBC without nodal disease on conventional imaging. However, 68Ga-FAP-2286 PET identified avid pelvic and paracaval lymph nodes (Fig. 5). Because of hearing loss and neuropathy, he was ineligible for cisplatin-based chemotherapy. Thus, he underwent upfront radical cystectomy with bilateral extended pelvic lymphadenectomy (up to the aortic bifurcation), an approach generally beyond the standard anatomic boundaries used for pelvic lymph node dissection, as pathology was anticipated to be aggressive. Final pathology revealed pT3aN3 disease with 12 of 38 lymph nodes involved. The nodal distribution confirmed the findings noted on 68Ga-FAP-2286 PET (not seen on conventional imaging), with involvement of the paracaval, right and left common iliac, right external iliac, and left obturator lymph nodes. The patient subsequently underwent adjuvant systemic immunotherapy given his nodal status (21) and remained disease-free at 8 mo after surgery.
78-y-old man presenting with lymph node–negative, localized MIBC on initial staging 68Ga-FAP-2286 PET (top and maximum-intensity projection at left), PET/CT (middle), and CT (bottom). Imaging revealed multiple paracaval and pelvic lymph nodes (denoted by arrows). Patient underwent radical cystectomy with extended lymph node dissection, which confirmed spread of cancer at time of surgery.
DISCUSSION
In this study, we applied 68Ga-FAP-2286, a novel peptide binder of FAP, to image patients with either metastatic or clinically localized urothelial cancer. In the metastatic cohort, 68Ga-FAP-2286 PET identified subcentimeter lymph nodes that would not otherwise meet RECIST criteria for malignancy. In the localized cohort, 68Ga-FAP-2286 PET confirmed the nodal status defined on conventional imaging and additionally identified a significant number of subcentimeter lymph nodes, leading to a change in clinical management for several patients.
We demonstrate that 68Ga-FAP-2286 PET was able to identify both false-positive and false-negative results on conventional imaging, with biopsy confirmation. For cases 1 and 2, the morbidity of cystectomy was avoided and a more appropriate treatment pursued. In the third case, 68Ga-FAP-2286 PET more accurately identified locally advanced or metastatic lymph nodes before surgery, leading to a modified surgical plan with extended lymph node dissection. Thus, in our cohorts with either localized or metastatic urothelial cancer,68Ga-FAP-2286 PET demonstrated the ability to stage local and metastatic disease more reliably than conventional imaging. We envision that 68Ga-FAP-2286 PET will ultimately play an important role in identifying patients with MIBC who may not benefit from radical surgery.
Our results meaningfully add to the growing body of studies on 68Ga-FAP-2286-based imaging in various tumor types (22)—studies that have shown several potential advantages over 18F-FDG PET. Consistent with a prior study using FAPI imaging in bladder cancer (20), 68Ga-FAP-2286 uptake was on average higher than 18F-FDG uptake. This feature leads to an improved tumor-to-background ratio and finer resolution of nodal disease, as we report in Supplemental Figure 1. Our results also highlight the ability of 68Ga-FAP-2286 PET to potentially address the long-standing challenge of improving sensitivity to define pelvic nodal metastasis using criteria beyond size or metabolism, with potentially less susceptibility to false positives (as highlighted in case 2). Finally, given the lack of patient preparation required the night before imaging, 68Ga-FAP-2286 PET may also be advantageous over 18F-FDG PET from the perspective of patient preparation and workflow.
Use of this imaging modality in bladder cancer more broadly highlights potential benefits in imaging specific cancers with unique cell-surface protein antigens, which may allow for more accurate and sensitive staging. Two relevant examples include prostate-specific membrane antigen PET, which is widely used in men with biochemically recurrent prostate cancer and has been shown to identify small metastases not otherwise seen on conventional imaging (23), and 89Zr-deferoxamine-girentuximab, a carbonic-anhydrase binder that more accurately stages renal cell carcinoma (24).
The generally high SUVmax and tumor-to-background ratio of 68Ga-FAP-2286 across all lesion sizes raises the possibility that 68Ga-FAP-2286 can be effective therapeutically. Previous studies using 90Y-FAPI-46 demonstrated safety and some efficacy in stabilizing advanced disease in patients with various malignancies, though at the expense of hematologic toxicity (25). It will be important to evaluate ongoing therapeutic studies targeting FAP in bladder cancer, including the metastatic bladder cohort of LUMIERE (17), as well as parallel studies of 177Lu-FAP-2286 reported in an initial cohort (26).
This study had several limitations, including the small sample size and lack of central pathology or masked radiology review. Moreover, this study was not specifically designed to detect false positives or false negatives using 68Ga-FAP-2286 PET. Specifically, the neoadjuvant treatment that some patients in the localized cohort had undergone before lymph node sampling during cystectomy would be expected to influence nodal status by potentially downstaging them (3). Finally, the results are more difficult to generalize to the broader population of bladder cancer patients because of the lack of histologic variants (e.g., squamous, neuroendocrine, or small cell). Nonetheless, a future larger prospective cohort addressing these concerns using 68Ga-FAP-2286 would be valuable.
CONCLUSION
68Ga-FAP-2286 is a promising FAP ligand for the initial staging of MIBC, a disease that is difficult to accurately stage with conventional imaging. 68Ga-FAP-2286 PET identifies metastatic lymph nodes smaller than 1 cm and may have higher specificity than 18F-FDG PET. Although these results need to be validated prospectively and in larger cohorts, 68Ga-FAP-2286 imaging may have an important role in initial staging, disease monitoring, and appropriate treatment selection for patients with MIBC.
DISCLOSURE
Thomas Hope has grant funding from Clovis Oncology (used to complete this study), Philips, GE Healthcare, Lantheus, Janssen, the Prostate Cancer Foundation, and the National Cancer Institute (R01CA235741 and R01CA212148). He received personal fees from Ipsen, Bayer, and BlueEarth Diagnostics and received fees from and has an equity interest in RayzeBio and Curium. Vadim Koshkin reports serving in a consulting or advisory role for AstraZeneca, Clovis, Janssen, Pfizer, EMD Serono, Seagen, Astellas, Dendreon, Guidepoint, GLG, and ExpertConnect; has received research funding for the institution from Endocyte, Nektar, Clovis, Janssen, and Taiho; and is supported by the Prostate Cancer Foundation. Terence Friedlander reports serving in a consulting or advisory role for AstraZeneca, Aadi Biosciences, Seagen, Merck, and Astellas and has received research funding for the institution from Roche-Genentech, Seagen, and Bristol-Meyers-Squibb. Sima Porten reports receiving research funding from Photocure, serving as a consultant for Stryker and Photocure, receiving an honorarium from Pacific Edge, and serving on the advisory board for Oncuria and AstraZeneca. No other potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: Is 68Ga-FAP-2286 PET an effective imaging modality in the initial staging of MIBC?
PERTINENT FINDINGS: 68Ga-FAP-2286 PET is effective at identifying bladder cancer that has spread outside the bladder, including small lesions that appear unremarkable on conventional imaging. In several cases, this imaging modality meaningfully influenced patient management.
IMPLICATIONS FOR PATIENT CARE: Imaging patients with newly diagnosed bladder cancer using 68Ga-FAP-2286 PET improves staging and potentially improves patient management.
Footnotes
↵* Contributed equally to this work.
Published online Jan. 11, 2024.
- © 2024 by the Society of Nuclear Medicine and Molecular Imaging.
REFERENCES
- Received for publication August 24, 2023.
- Revision received November 7, 2023.