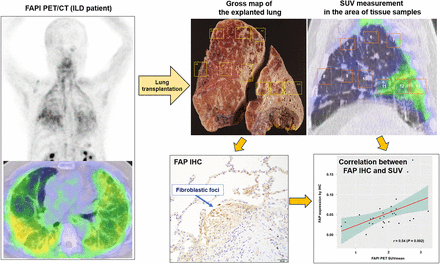
Visual Abstract
Abstract
Recent studies have demonstrated promising results of fibroblast activation protein (FAP) inhibitor (FAPI) PET in prognosticating and monitoring interstitial lung diseases (ILDs). As a first step toward successful translation, our primary aim was to validate the FAPI PET uptake through immunohistochemistry in patients with advanced ILD who underwent lung transplantation after a FAPI PET scan. Methods: This is a preliminary analysis of a single-center, open-label, single-arm, prospective exploratory biodistribution study of 68Ga-FAPI-46 PET imaging in patients with ILD (NCT05365802). Patients with ILD confirmed by high-resolution CT and scheduled for lung transplant were included. Tissue samples of explanted lungs were obtained from both the central and peripheral lung parenchyma of each lobe. Additional samples were obtained from areas of the lung corresponding to regions of FAPI PET activity. Immunohistochemical staining was performed with an anti-FAP antibody. Percentages of FAP immunohistochemistry-positive area were measured semiautomatically using QuPath software. SUVs in the areas of pathologic samples were measured on FAPI PET/CT by referencing the gross photomap of the explanted lung. A Spearman correlation coefficient test was used to assess the relationship between FAPI PET uptake and FAP immunohistochemical expression in each specimen. Results: Four patients with advanced ILD who underwent FAPI PET/CT before lung transplantation were included. The types of ILD were idiopathic pulmonary fibrosis (n = 2), rheumatoid arthritis–associated ILD (n = 1), and nonspecific interstitial pneumonia (n = 1). FAPI uptake was visualized mainly in the fibrotic area on CT. Twenty-nine surgical pathology samples from 3 patients were analyzed. FAP staining was predominantly positive in fibroblastic foci. FAPI PET SUVmax and SUVmean showed a positive correlation with the immunohistochemical FAP expression score (SUVmax: r = 0.57, P = 0.001; SUVmean: r = 0.54, P = 0.002). Conclusion: In this analysis conducted in patients who underwent lung transplantation after a FAPI PET scan, FAPI PET uptake was positively correlated with FAP immunohistochemistry. These findings provide a rationale for further investigation of FAPI PET as a potential imaging biomarker for ILD.
Interstitial lung disease (ILD) is a heterogeneous group of diseases characterized by inflammation and fibrosis of the lung parenchyma (1). Progressive fibrosing ILD is a phenotype in which patients continue to progress despite conventional treatment directed at the underlying diagnosis (2). An accurate diagnosis is important to ensure that treatment is optimized before progressive fibrosis can be ascertained. However, it is difficult to predict which patients will develop a progressive fibrosing ILD phenotype at the time of diagnosis; thus, identifying biomarkers to improve risk stratification is critical (2).
Migration and activation of quiescent fibroblasts and the resulting excessive production of the extracellular matrix accelerate the fibrosing process (3). Radiographic assessment of fibroblast activation may serve as a surrogate measure of fibroblast activity in patients with ILD and help to distinguish patients with progressive versus stable ILD. Fibroblast activation protein (FAP) is linked to multiple human pathologies including fibrosis and cancer, and FAP expression is associated with severe and progressive disease (4). In murine models of ILD, the degree of FAP expression was associated with disease severity (5,6). The advent of FAP inhibitors (FAPIs) labeled with radioisotopes (e.g., 68Ga or 18F) has enabled PET imaging of FAP (7). Recent studies have shown promising results of FAPI PET in the imaging and monitoring of ILDs, such as idiopathic pulmonary fibrosis (IPF) and scleroderma-associated ILD (8–11). However, to the best of our knowledge, a study evaluating direct radiologic–pathologic correlation between FAPI PET uptake and FAP expression is lacking.
Establishing the correlation between FAP expression and FAPI PET uptake holds promise for precision diagnostics in ILD, potentially improving prognosis and survival outcomes for patients facing this challenging respiratory condition. As a first step toward successful clinical translation, our primary aim was to validate the biodistribution of FAPI PET uptake through histopathologic and immunohistochemical analyses of explanted lung tissue. In addition, since high-resolution CT (HRCT) is indispensable in the management of ILD, our secondary objective (post hoc) was to explore the correlation of FAPI PET uptake to HRCT.
MATERIALS AND METHODS
Study Design and Participants
This is a preliminary analysis of a single-center, open-label, single-arm, prospective exploratory study of 68Ga-FAPI-46 PET imaging conducted under the Radioactive Drug Research Committee Program (title 21 of Code of Federal Regulations, section 361.1). Patients with ILD, confirmed by HRCT, who were scheduled to undergo cryobiopsy or transplantation of the affected lung were eligible (inclusion and exclusion criteria are available in Supplemental Table 1 (12–18); supplemental materials are available at http://jnm.snmjournals.org). Imaging findings of FAPI PET/CT did not impact the therapy management. This study was approved by the UCLA Institutional Review Board (21-000678) and was registered on ClinicalTrials.gov (NCT05365802). All patients provided oral and written informed consent. The primary objective was to correlate the FAPI PET uptake with FAP expression by immunohistochemistry. The secondary objective, as a post hoc analysis, was to correlate FAPI PET with HRCT.
68Ga-FAPI-46 PET/CT
68Ga-FAPI-46 was used as the FAP-targeted radioligand (19). The median injected activity was 185 MBq (range, 130–192 MBq). The mean uptake time was 64.6 min (range, 61–71 min). PET/CT images were acquired on either a Siemens Biograph 64 mCT (Siemens Healthineers) (n = 2) or a Siemens Biograph TruePoint (Siemens Healthineers) (n = 2) without respiratory synchronization. PET reconstruction parameters were as follows: Biograph 64 mCT (3-dimensional ordered-subset expectation maximization, 2 iterations, 24 subsets); Biograph TruePoint (2-dimensional ordered-subset expectation maximization, 2 iterations, 8 subsets). Unenhanced CT (120 kV, 80 mAs) was performed for attenuation correction and anatomic correlation. PET/CT images covered midthigh to vertex and were taken with the patient in a supine position and their arms up and without breath hold (free breathing).
HRCT
HRCT images obtained most recently before the FAPI PET scans were used for correlation with FAPI PET/CT. HRCT volumetric scans were obtained in 1-mm thin slices in the supine and prone position. Both inspiratory and expiratory CT images were obtained with breath hold. Inspiratory CT in the supine position was used for the analysis. Four CT scanners were used (Siemens SOMATOM Definition Flash: 120 kV, 311 mAs; Siemens Sensation 64: 120 kV, 240 mAs; Siemens SOMATOM Definition AS: 120 kV, 238 mAs: Siemens SOMATOM Force: 120 kv, 120 mAs).
Image Analysis
Visual and quantitative analyses of the FAPI PET/CT were performed by 1 dual–board-certified physician in radiology and nuclear medicine. The reader was unmasked to clinical characteristics (e.g., type of ILD, laboratory data, pulmonary function test) but masked to the immunohistochemistry results.
Histopathology and FAP Immunohistochemistry
Tissue samples of explanted lungs were obtained from both the central and the peripheral lung parenchyma of each lobe (3 lobes on the right and 2 lobes on the left), per our institution’s routine standard-of-care protocol. Additional samples were obtained from areas of the lung corresponding to regions of FAPI PET activity on pretransplant imaging studies. Immunohistochemical staining for FAP (Abcam anti-FAP, α-antibody [EPR20021]) using 3,3′-diaminobenzidine tetrahydrochloride was performed on formalin-fixed paraffin-embedded tissue by the UCLA Translational Pathology Core Laboratory.
Quantification of FAP Immunohistochemical Expression
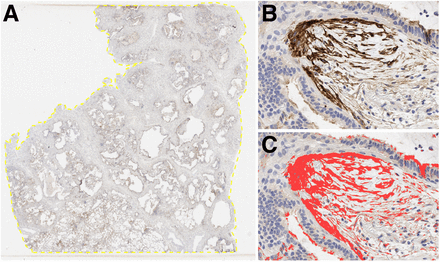
The FAP-stained slides were scanned at ×20 using an Aperio AT2 whole slide scanner (Leica Biosystems). The digitally scanned slides were analyzed (Fig. 1) using QuPath software, version 0.3.2 (20). The entire tissue fragment was manually traced using the mouse; the entire tissue area was automatically calculated. The area of FAP positivity was measured using the thresholder tool with the following parameters: resolution = full (0.50 μm/pixel), channel = 3,3′-diaminobenzidine tetrahydrochloride, prefilter = gaussian, smoothing σ = 0, and threshold = 0.25. The ratios between immunohistochemistry-positive areas and the entire tissue areas were calculated as a percentage.
FAP-stained slides were analyzed using QuPath software. (A) Entire tissue fragment was manually outlined using mouse (yellow line); area was automatically calculated. (B) FAP-positive, brown-stained areas were largely localized to fibroblast foci. (C) FAP-positive areas (red) were measured using thresholder tool.
Correlation of FAP PET to FAP Immunohistochemistry
Rectangle-shaped regions of interest, adjusted to the same size as the pathologic specimens, were placed on the FAPI PET/CT sections. The regions of interest were set at the same cross section as the immunohistochemical sections. They were placed at the locations where the immunohistochemical specimens were obtained by referring to the pathologic map (Fig. 2). SUVmax and SUVmean of the regions of interest (i.e., areas of pathologic specimens) were measured using sagittal PET/CT slices.
(A) Gross pathologic photograph of right explanted lung in patient 2 (parasagittal, 3 cm from hilum). Rectangles indicate locations from which pathologic specimens were obtained. (B) Sagittal FAPI PET/CT image. Slice location was set same as that of pathologic map. Rectangular regions of interest were placed to measure SUVmax and SUVmean of pathologic specimen area, referencing pathologic map. Numbers in regions of interest represent corresponding specimen numbers.
Statistical Analysis
R software and Stata software were used for statistical analysis. Two-tailed P values of less than 0.05 were considered significant. A Spearman correlation coefficient test was used to assess the relationship between FAPI PET uptake (i.e., SUVmean and SUVmax) and FAP immunohistochemistry (i.e., positive area in percent).
RESULTS
Patients
Between November 2021 and April 2022, 4 patients with advanced ILD who underwent FAPI PET/CT before lung transplantation were enrolled in the study. Patient characteristics and radiologic images are shown in Table 1. The types of ILD included probable IPF or usual interstitial pneumonia (n = 2), rheumatoid arthritis–associated ILD (n = 1), and nonspecific interstitial pneumonia (vs. chronic hypersensitivity pneumonitis) (n = 1). Three patients underwent right lung transplantation, whereas 1 patient (patient 3) underwent left lung transplantation because she had previously undergone right lung transplantation. The median time interval between HRCT and PET/CT was 158 d (range, 6–188 d). The median time interval between PET/CT and lung transplantation was 32 d (range, 5–50 d). Patient radiologic images are shown in Figures 3–6.
Patient Characteristics
Patient 1: 68-y-old man with rheumatoid arthritis–associated ILD. (A) HRCT images showed progression of fibrosis over time. (B) Lung perfusion scans with 99mTc macroaggregated albumin showed heterogeneous distribution throughout lung (60% uptake in right, 40% uptake in left). (C) FAPI PET maximum-intensity projection and PET/CT showed uptake in lung parenchyma predominantly in subpleural area (SUVmax, 3.5). (D) Hematoxylin and eosin (H&E) and immunohistochemical staining of FAP of explanted lung specimen (right lower lobe, peripheral). ANT = anterior; POST = posterior.
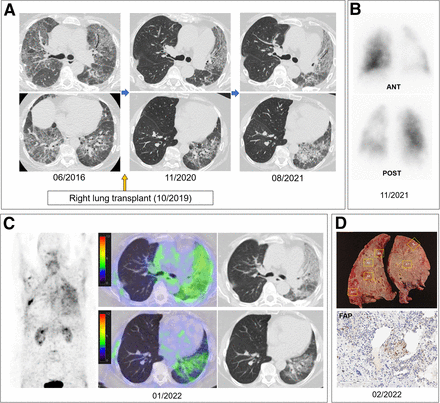
Patient 2: 66-y-old man with probable IPF or usual interstitial pneumonia. (A) HRCT images showed progression of fibrosis over time. (B) FAPI PET maximum-intensity projection and PET/CT showed uptake (SUVmax, 5.1) predominantly in lower basal regions of lung. (C) Gross pathology and immunohistochemical staining of FAP (right middle lobe, peripheral) of explanted lung.
Patient 3: 60-y-old woman with nonspecific interstitial pneuomonia (vs. chronic hypersensitivity pneumonitis) who had history of right lung transplantation. (A) HRCT images showed gradual progression of fibrosis in left lung, whereas transplanted right lung showed no significant abnormalities. (B) Lung perfusion scans with 99mTc macroaggregated albumin showed decreased distribution in left lung (74% uptake in right, 26% uptake in left). (C) FAPI PET maximum-intensity projection and PET/CT showed uptake (SUVmax, 4.5) in left lung but not in transplanted right lung. (D) Gross pathology and immunohistochemical staining of FAP (left lower lobe, peripheral) of explanted lung. ANT = anterior; POST = posterior.
Patient 4: 68-y-old woman with probable IPF or usual interstitial pneumonia. (A) HRCT images showed mild progression of pulmonary fibrosis. (B) Lung perfusion scans with 99mTc macroaggregated albumin showed no significant abnormalities (54% uptake in right, 46% uptake in left). (C) FAPI PET maximum-intensity projection and PET/CT showed uptake (SUVmax, 4.2) predominantly in subpleural area of both lungs. (D) Gross pathology and immunohistochemical staining of FAP (right lower lobe, peripheral) of explanted lung. ANT = anterior; POST = posterior.
68Ga-FAPI-46 PET/CT
FAPI uptake was predominantly observed in ground-glass opacities (GGO) and fibrotic areas (e.g., reticular opacities, consolidations) rather than in the normal lung. The mean ± SD SUVmax were 4.2 ± 0.7 in the whole lung, 3.6 ± 1.2 in the right upper lobe, 3.3 ± 1.3 in the right middle lobe, 3.6 ± 1.1 in the right lower lobe, 3.7 ± 0.5 in the left upper lobe, and 3.6 ± 0.8 in the left lower lobe.
Correlation with Immunohistochemistry
FAP immunohistochemical staining was predominantly positive in fibroblastic foci (Supplemental Fig. 1). For the correlation analysis between FAP immunohistochemistry and PET uptake, 29 pathology specimens from 3 patients were used (n = 9, 8, and 12 from patients 2, 3, and 4, respectively). The pathology specimens from patient 1 could not be used for correlation analysis because a gross pathology map was not generated because of a clerical error. Both FAPI PET SUVmean (r = 0.54, P = 0.002) and SUVmax (r = 0.57, P = 0.001) showed a moderate positive correlation with immunohistochemical FAP expression (Fig. 7).
Scatter plots of FAP expression by immunohistochemistry (IHC) (percent positive area) in pathologic specimens and FAPI PET uptake: SUVmax (A), SUVmean (B) in corresponding lung regions. Red line is regression line; green area is CI; r represents correlation coefficient.
DISCUSSION
Establishing a correlation between FAPI PET uptake and pathologic FAP expression holds great promise for the early diagnosis and prognosis of ILD. However, to the best of our knowledge, studies directly comparing PET uptake and FAP immunohistochemical expressions are lacking. To confirm the relevance of FAP PET in assessing fibroblast activity in ILD, this exploratory biodistribution study was conducted using a clinico-histopathologic correlation approach in patients who subsequently underwent lung transplantation after a FAP PET scan. In addition, correlation with HRCT was conducted as a post hoc analysis.
Röhrich et al. (8) first reported 68Ga-FAPI-46 PET uptake in patients with various ILDs (n = 15). In their study, 4 lung fibrosis specimens obtained by biopsy were available and FAP positivity was confirmed mainly in the transition zone between lung tissue and fibrotic areas. However, the biopsy areas of the specimens were not specified, and direct comparisons with FAPI PET uptake were not conducted. Later, Yang et al. (9) analyzed 83 patients (n = 20 with IPF and n = 63 with non-IPF) who underwent 68Ga-FAPI-04 PET, with lung biopsy specimens available in 33 patients. They confirmed that FAP expression was upregulated in the early phase of lung fibroblast activation, whereas the correlation between pathologic FAP expression and PET uptake was not evaluated.
In our study, FAP expression was predominantly observed in the fibroblastic foci. This is consistent with the previous study by Yang et al., in which a positive correlation between the extent of FAP expression and the pathologic grades of fibrotic foci was found (9). Fibroblastic foci represent aggregates of collagen-producing activated fibroblasts or myofibroblasts, and their extent correlates with a worse clinical outcome in ILD (5,21,22). It is also worth noting that a higher volume of FAPI PET uptake in the lung has also been reported to be associated with lung function decline in ILD (9,10).
A correlation between FAPI PET uptake (SUVmean and SUVmax) and the extent of FAP expression was moderate (SUVmax: r = 0.57, SUVmean: r = 0.54). The correlation might have been stronger if the patients were not in the end-stage of pulmonary fibrosis. Some experimental studies have found that FAP expression is induced in the early phase of lung fibroblast activation rather than in the late phase of lung fibrosis (9,23). This is consistent with our pathologic observation of lower FAP expression in late-stage fibrotic areas like honeycomb. Liu et al. reported lower 99mTc-FAPI uptake in areas of severe lung fibrosis defined by HRCT (24). Since FAPI PET uptake is affected not only by FAP expression but also by the vasculature (25,26), decreased blood flow in end-stage fibrosis may also contribute to the lower FAPI uptake.
The FAPI PET SUVmean showed a positive correlation with HRCT ILD scores. This is consistent with previous studies. Mori et al. reported a positive correlation between whole-lung 18F-FAPI-74 PET SUVmean (defined as an area >45% of SUVmax) and Hounsfield units in the corresponding area in patients with IPF (11). Bergmann et al. reported similar findings in patients with systemic sclerosis-associated ILD, in which whole-lung 68Ga-FAPI-04 SUVmean (defined as an area >45% of SUVmax) correlated with disease extent as assessed visually by HRCT (10). Röhrich et al. compared 68Ga-FAPI-46 PET lobar SUVmean and HRCT indices (fibrosis and GGO) calculated on the basis of Hounsfield units (8). They found a positive correlation of SUVmean with the fibrosis index (r = 0.57), whereas a negative correlation was observed with the GGO index (r = −0.44). In contrast, in our study, FAPI uptake was positively correlated with the GGO-based HRCT score. This discrepancy may be due to differences in the methods used to calculate the GGO score (Hounsfield units vs. machine learning) and different patient populations. The pathologic background of GGO is diverse, and GGO may represent not only early fibrosis but also inflammatory processes (27).
The study has several limitations. First, the small patient cohort should be mentioned. However, all patients underwent transplantation, and explanted lungs were accessible for radiologic–pathologic correlation. Thus, a total of 29 pathologic lung specimens from 3 patients with traceable sites on CT were available, enabling statistical significance in the correlation analysis between FAPI PET uptake and FAP expression by immunohistochemistry. Second, the explanted lungs may have shrunk, which could limit the accuracy of the anatomic correlation between FAPI PET and the gross pathologic map. Third, the use of antifibrotic drugs may have influenced the FAPI PET uptake and FAP expression by immunohistochemistry. Two patients were on nintedanib at the time of PET and transplantation, which may have resulted in decreased FAPI uptake (10). Further studies with larger populations, including cases with early fibrosis, and studies investigating the FAPI PET uptake before and after antifibrotic drug administration are warranted.
CONCLUSION
This preliminary analysis of this prospective exploratory biodistribution study conducted in 4 patients with ILD who underwent lung transplantation after a FAPI PET scan identified a positive radiologic–pathologic correlation between FAPI PET uptake and FAP expression assessed by immunohistochemistry. FAP expression was concentrated in fibrotic foci, indicating active fibrogenesis. These findings provide a rationale for further investigation of FAPI PET as a potential imaging biomarker for ILD.
DISCLOSURE
Johannes Czernin is a founder of SOFIE Bioscience, a founder of Trethera, and is on the medical advisory board of RayzeBio. No other potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: Does FAPI PET uptake reflect pathologic FAP expression in patients with ILD?
PERTINENT FINDINGS: The study found a positive correlation between FAPI PET SUVs and the extent of immunohistochemical FAP expression in explanted lungs. FAP expression was concentrated in fibrotic foci, indicating active fibrogenesis.
IMPLICATIONS FOR PATIENT CARE: The findings support the potential of FAPI PET as an imaging biomarker for ILD, correlating well with histopathologic FAP expression and suggesting its utility for assessing fibrotic activity in ILD.
ACKNOWLEDGMENTS
We thank the UCLA Translational Pathology Core Laboratory for its assistance with whole-slide imaging.
Footnotes
Published online Oct. 3, 2024.
- © 2024 by the Society of Nuclear Medicine and Molecular Imaging.
REFERENCES
- Received for publication July 3, 2024.
- Accepted for publication September 19, 2024.